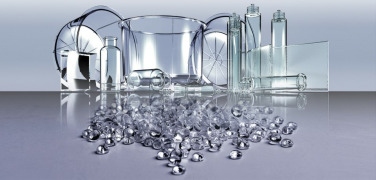
TOPAS COC

Product Description
TOPAS Advanced Polymers GmbH

-
DE
-
2021On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
TOPAS Advanced Polymers GmbH

-
DE
-
2021On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from TOPAS Advanced Polymers GmbH (3)
-
Product TOPAS® COC Grades (Cyclic Olefin Copolymer)
Cyclic olefin copolymers are a class of polymeric materials with property profiles which can be varied over a wide range during polymerization.
Performance benefits are:
• Low density • High transparency • Low birefringence • Extremely low water absorption • E... -
Product TOPAS® COC Elastomer (Cyclic Olefin Copolymer)
TOPAS COC Elastomer is a variant of COC that offer a unique set of properties because it’s a pure polymer
• Ultra low level of extractables • Good mechanical properties (as a static gasket) • Excellent static leakage performance • Good moisture ingress barrier -
Product DURACON POM PM Series
Polyplastics has commercialized DURACON® POM (Polyoxymethylene / Acetal) PM Series for medical applications.In the medical device marketplace, manufacturers and end users demand high quality materials from reliable suppliers. As the world’s leading manufacturer of POM, Polyplastics now adds DURACON®&n...
TOPAS Advanced Polymers GmbH resources (2)
-
Brochure Medical
The combination of high transparency, higher shatter resistance than glass and excellent moisture barrier makes TOPAS® COC particularly attractive for prefillable pharmaceutical primary packaging. The material is also characterized by high purity and excellent biocompatibility.
Examples of applications include prefillable syringes and vials as well as a variety of prefillable containers that can be used, for example, as part of injection systems.
Injection molding and injection blow molding/stretch blow molding are typical manufacturing processes for this type of pharmaceutical packaging.
In addition, TOPAS® COC is increasingly used for pharmaceutical blister packaging due to its high transparency, biocompatibility and excellent moisture barrier. -
Brochure Packaging
Packaging for medicines and medical devices must meet such demanding criteria as biocompatibility, protection against external influences, optimum shelf life, clear labeling and easy handling and removal. Blister packs have become increasingly popular for medicines in tablet form because they are easy and safe for patients to use and help them to comply with dosage recommendations. Modern medical devices often need to be protected from moisture, while clarity is desired for easy identification and quality control.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance