Tinefcon
Product Description
Invex Health
-
IN
-
2022On CPHI since
-
4Certificates
-
25 - 49Employees
Company types
Primary activities
Categories
Specifications
Invex Health
-
IN
-
2022On CPHI since
-
4Certificates
-
25 - 49Employees
Company types
Primary activities
More Products from Invex Health (6)
-
Product Biosimilars
Products: Trastuzumab Bevacizumab Rituximab Adalimumab Etanercept Tenecteplase Plant Approval: EUGMP, WHO GMP, Philippines FDA & TMMDA -
Product Blood Products
Products: Human Albumin IVIG Dried Factor VIII Fraction Erythropoietin Darbepoetin alfa Injection Filgrastim Human Anti-D Immunoglobulin Plant Approval: WHOGMP, Kenya, Ethiopia -
Product Peg-asparaginase & other oncology products
• Vandetanib tablets • L - Asparaginase injection • Peg - Asparaginase injection • Thiotepa injection • Melphalan injection • Paclitaxel Albumin bound nanoparticle injection • Plerixafor injection and more... -
Product Bevacizumab & other Biosimilars
• Trastuzumab • Rituximab • Adalimumab • Etanercept • Tenecteplase & More -
Product Human albumin & other Blood products
• Human Albumin IVIG • Dried Factor VIII Fraction • Erythropoietin • Darbepoetin alfa Injection • Filgrastim • Human Anti-D Immunoglobulin -
Product Cardio-Diabetics
• Rifaximin tablets • Rivaroxaban tablets • Apixaban tablets and more.
Invex Health resources (4)
-
Brochure Product list (ROW market)
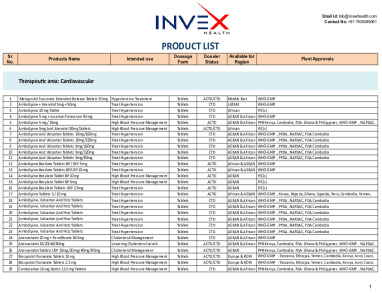
These are the products available for ROW markets with dossier status. -
Brochure Product list - Stringent Regulated Markets
These are the products available for stringent regulated markets with Dossier status. -
Brochure Special Product Range (Blood products & Biosimilars)
Available only for special import opportunities and selected ROW market registration -
Brochure Invex Health e-brochure
Invex Health is a global healthcare company offering a portfolio of 600+ products across Pharmaceuticals, Nutraceuticals, Dermo-cosmetics, and Medical Devices. We serve markets in SEA, LATAM, CIS, North America, and Africa backed by CMO plant approvals from top regulatory bodies like EU-GMP, PIC/S, UK-MHRA, WHO-GMP, T-FDA, NAFDAC, ANVISA, INVIMA, and more.
We cover key therapeutic areas like Oncology, Biosimilars, Cardiovascular, Anti-diabetics, Dermatology, and Neurology & more.
Our Services include:
Product RegistrationTender ParticipationTechnology TransferClinical Trial Supply
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance