The PSCI publishes Materiality Assessment of the Pharma Supply Chain
.png)
In the latest instalment of the PSCI blog on CPHI Online, The PSCI introduces the 2024 PSCI Supply Chain Materiality Assessment, the first public materialty assessment of the pharmaceutical supply chain they have worked on.
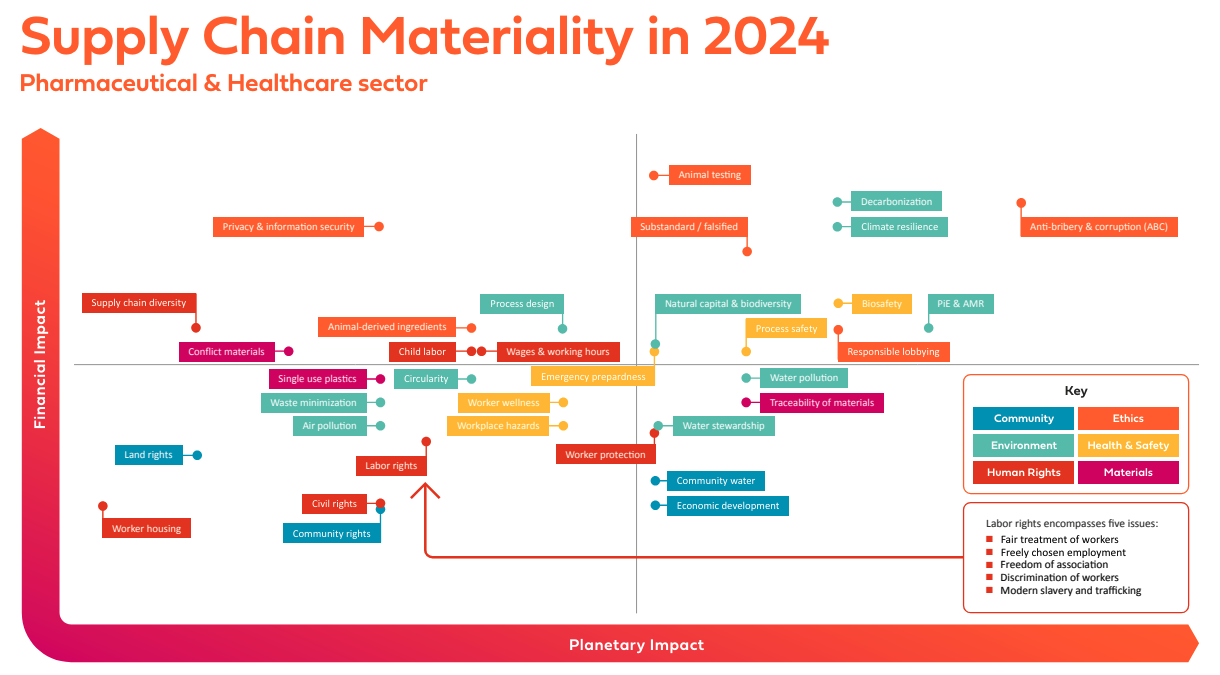
The 2024 PSCI Supply Chain Materiality Assessment focused on supply chains for the Pharmaceutical and Healthcare industries, taking into account the views of its own members, as well as a range of sources from outside the PSCI.
These assessments are conducted periodically by the PSCI, but this year the association decided to apply a double materiality approach, based on the methodology published by the European Financial Reporting Group (EFRAG), that scores issues against two categories: business or financial impacts, and planetary impacts.
The assessment results contain a number of key findings divided into six categories: ethics, human rights, health & safety, environment, materials, and community.
There are several notable new inclusions this year, including:
Ethics: responsible engagement on policy (lobbying) & substandard and falsified medicines
Human Rights: diversity and inclusion & housing conditions
Health & Safety: workplace hazards & biosafety
Environment: climate change resilience
Materials: traceability of raw materials
Community: communities’ human rights
Reflecting on the assessment, PSCI Chair & Global EH&S Supplier Operations and Business Development Lead at Viatris, Dr Deirdre O’Reilly, said:
“New regulations and investor demands are raising the bar for company reporting on supply chain responsibility. This year's assessment highlights the growing importance of robust supply chain programmes, encompassing a wider range of issues. It also reflects the changing nature of the industry’s products and markets.”
The assessment is intended to represent the sector as a whole, and is not reflective of each individual company. Pharma is a diverse industry and material issues for individual businesses will differ depending on a variety of factors like product mix, geography, ownership, etc. A full double materiality review would typically include rigorous quantification of impacts. As this is challenging to do at a sector level, this assessment from the PSCI used desk research along with member inputs to score issues using a framework aligned with EFRAG’s double materiality guidance.
Research resources for the assessment included:
- The latest legislation, reporting frameworks, and investor indices;
- Media reports, NGO activity, and other relevant reports and research;
- External stakeholder input including PSCI’s advisory panel and other partner organisations;
Finally, another vital part of the process was member inputs. These inputs came through ongoing and regular discussions within the PSCI’s own topic teams, as well as through the 2023 member consultation across all PSCI Principles topics. Throughout the assessment and scoring, a patient lens was applied, considering how patients could be impacted by substantially positive or negative handling of each issue.
The PSCI intends to use the results of this assessment to ensure that new and emerging topics are considered (and top issues given due focus in their activities), that the position of important stakeholders on supply chain responsibility topics is systematically understood, and finally that PSCI members’ responsibility programmes are covering all material issues.
For the 2024 PSCI Supply Chain Materiality Assessment, click here.
Related News
-
News CPHI Pharma Awards 2024: Meet the winners from the CPHI Celebration
This year we had a lot to celebrate, the 35th Anniversary of CPHI, and our esteemed award winners, of which we included two additional categories this year, the Future Leader award, and Woman of the Year award. -
News Women in Pharma: C-Suite Journeys in Leading Diversity
In this CPHI Milan special of our monthly series, we sit down with our panel of C-suite executives speaking on the ‘Leading with Diversity: The CEO Journey’ panel at this year’s show. -
News The BIOSECURE Act: implications for the pharma supply chain
On September 9, 2024, the US House of Representatives voted to pass the bill titled the BIOSECURE Act (the Act), which lists several Chinese companies in the pharmaceutical supply chain. The Act will prohibit American companies from contracting or doin... -
Sponsored Content CPHI Online Trend Report: How can flow chemistry help businesses achieve their sustainability goals?
In our latest CPHI Online Trend Report, we partner with Asymchem to understand the innovative potential of flow chemistry for API manufacturing, especially in regards to meeting sustainability goals. -
News CPHI Milan Speaker Spotlight: CDMO relations with Pharma and Start-Ups
In the run-up to CPHI Milan, we sit down with some of the experts and thought-leaders speaking at this year’s conferences. -
News Women in Pharma: Advocating for trans healthcare in pharma
In our monthly series on women in the pharmaceutical industry, we interview leading experts in the pharmaceutical supply and value chain to discuss the importance of gender diversity in healthcare, the workplace, and beyond. -
News A Day in the Life of a Vice President in R&D & Engineering
In the Day in the Life of Series, we've already had the chance to get to know a range of people in various roles in the pharma industry. In the latest interview we get a glimpse into the R&D side of things from Jennifer Sorrells, Vice Presiden... -
News CPHI Podcast Series: analysing supplier audits with the PSCI
This episode of the CPHI Podcast Series, hosted by Digital Editor Lucy Chard, goes through the results from the recent audits from the PSCI conducted on suppliers across the pharmaceutical industry, looking into ESG outcomes.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






.png)

