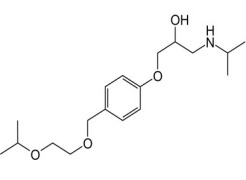
TETRACYCLINE
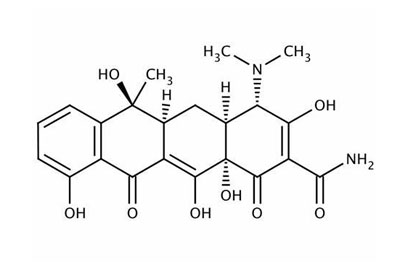
Product Description
SUANFARMA Group

-
ES
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
SUANFARMA Group

-
ES
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from SUANFARMA Group (28)
-
Product TIAMULIN
Antibiotic -
Product TRIMEBUTINE MALEATE
ANTIEMETIC -
Product VALSARTAN
ANTIHYPERTENSIVE -
Product LYMECYCLINE
ANTIBIOTIC -
Product MINOCYCLINE
ANTIBIOTIC -
Product OXYTETRACYCLINE CA
ANTIBIOTIC -
Product BISOPROLOL FUMARATE
ANTITUSSIVE -
Product ANASTROZOLE
ONCOLOGY -
Product PIPERACILLIN + TAZOBACTAM
ANTIBIOTIC -
Product ENOXAPARIN SODIUM
ANTICOAGULANT -
Product PREGABALIN
ANTIEPILEPTIC -
Product PIRFENIDONE
ANTIINFLAMATORY, ANTIRHEUMATIC
SUANFARMA Group resources (2)
-
Video Revolutionizing Pharma Manufacturing With TT&GO® Technology Transfer
In the rapidly evolving landscape of pharmaceutical manufacturing, the TT&GO® platform by Suanfarma CDMO stands out as a revolutionary solution for technology transfer. This session will explore into the unique methodology of TT&GO®, which integrates rigorous, systematic procedures grounded in risk-based analysis to facilitate seamless technology transfers. Methodological rigor and flexibility: Understanding the systematic approach of TT&GO® that applies to various phases of the product lifecycle, ensuring adaptability and compliance with Good Manufacturing Practices (GMP). Risk management and cost efficiency: Analyzing how TT&GO®'s proactive risk assessment and mitigation strategies lead to reduced failure rates and cost savings, empowering clients to pursue ambitious project timelines with minimized risks. Real-world applications and outcomes: Presenting case studies and success stories of TT&GO® in action, highlighting the platform's impact on accelerating commercial launches and enhancing market competitiveness. Attendees of this session can expect to leave with a comprehensive understanding of how advanced technology transfer methodologies like TT&GO® can be leveraged to optimize pharmaceutical manufacturing processes, ensuring higher efficiency, reduced risk, and better overall outcomes. -
Video Suanfarma, At the Core of a Better Life
Suanfarma is a key player in the life sciences sector, specializing in the development of active pharmaceutical ingredients (APIs) and contract development and manufacturing (CDMO) services. Since 1993, Suanfarma has been dedicated to enhancing global health by improving access to affordable medicines. We operate advanced facilities in Portugal and Italy, and supports over 400 laboratories worldwide through a robust network of 12 commercial offices.
Suanfarma’s proprietary APIs are developed using cutting-edge techniques, including AI and Flow Chemistry, ensuring high standards and GMP compliance. We are committed to sustainability and global health, offering innovative solutions for High Potency APIs and expanding fermentation and chemical synthesis capacities to meet growing global demand.
Our customer-centric approach and strong focus on quality makes us a trusted partner in the pharmaceutical industry.
...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance














-file153323.png)
