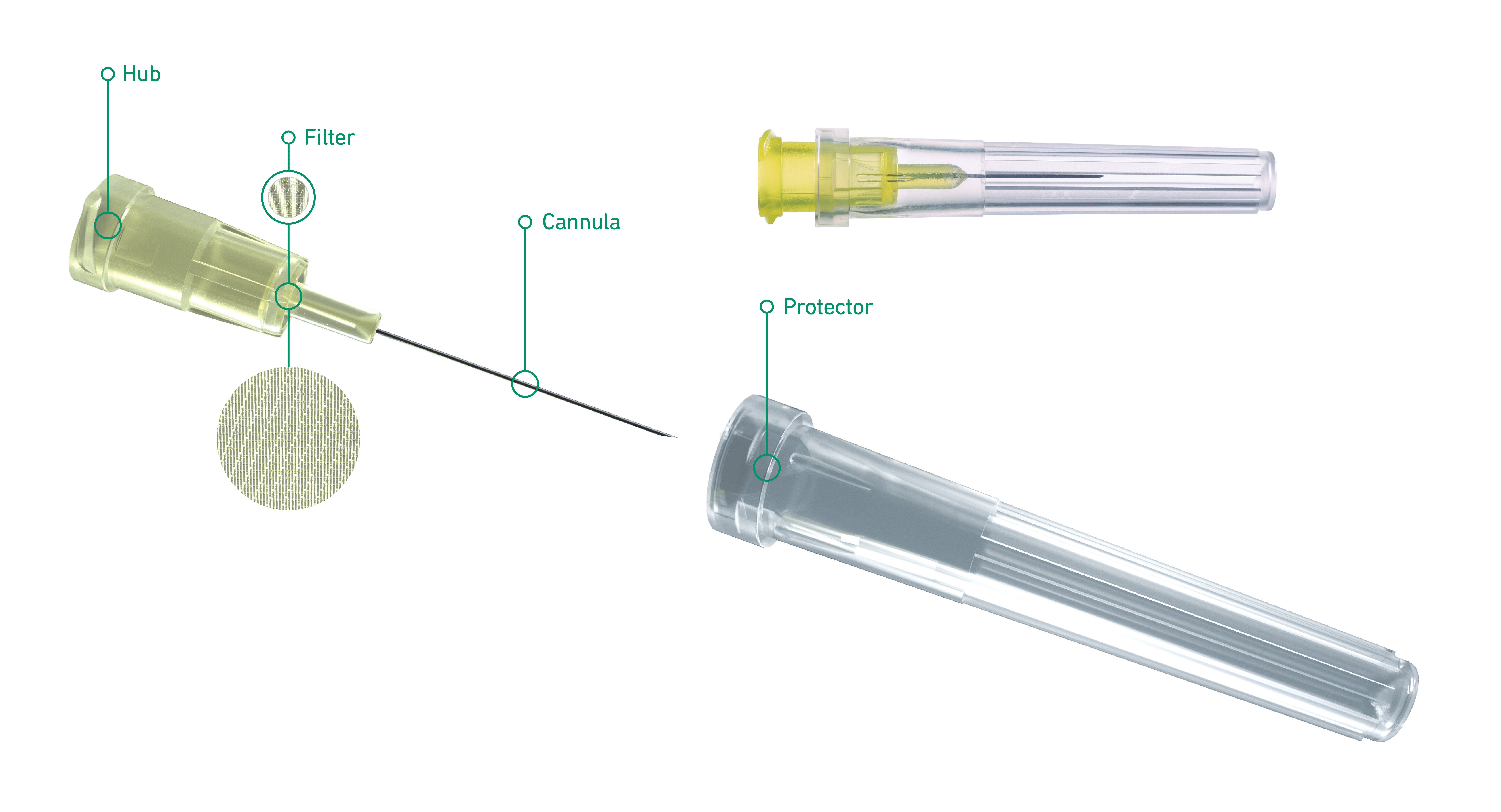
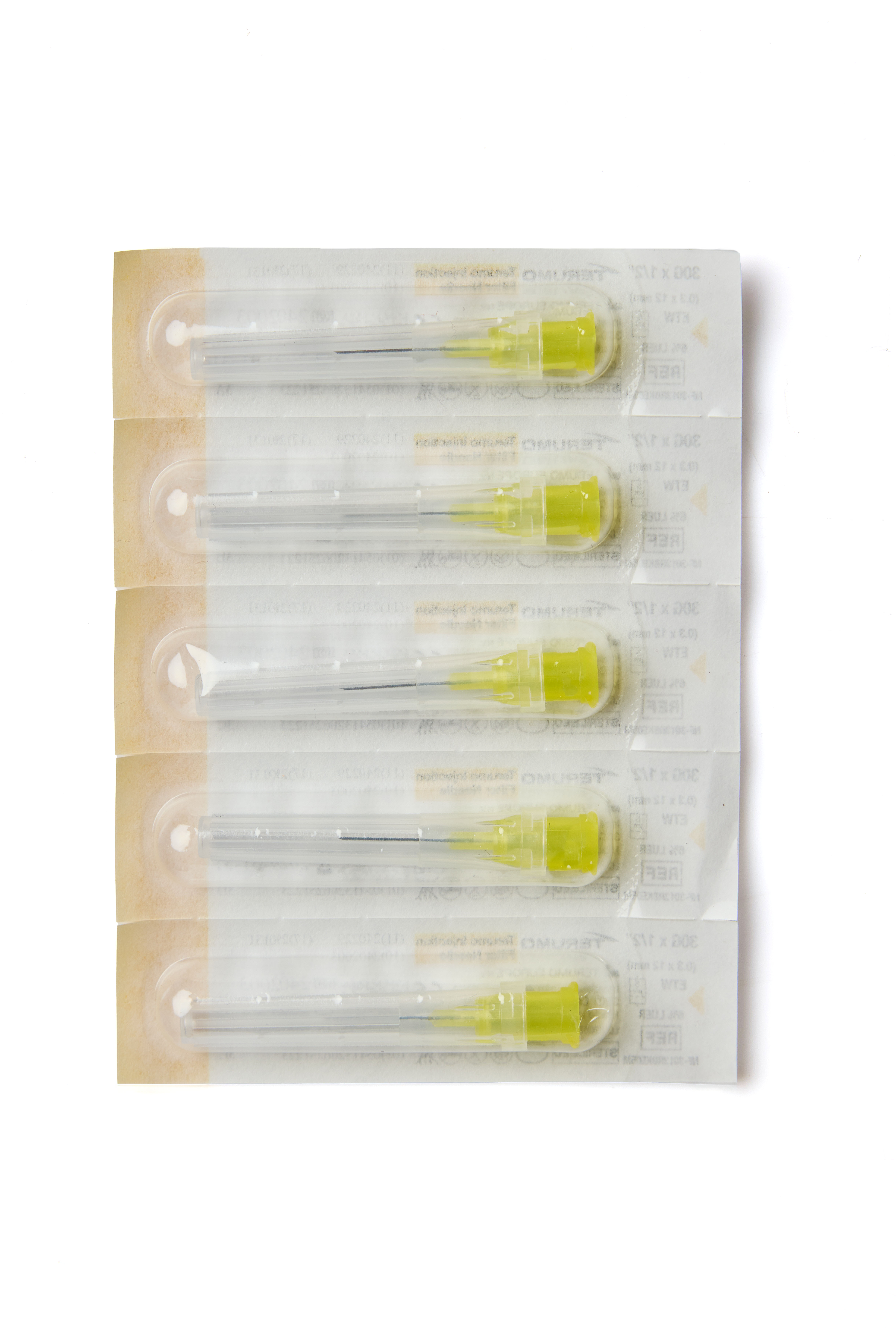
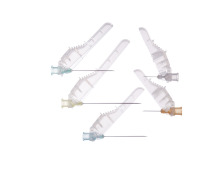
Terumo Injection Filter Needle
Product Description
TERUMO

-
BE
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
TERUMO

-
BE
-
2015On CPHI since
-
3Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from TERUMO (11)
-
Product SurGuard™2 Safety Hypodermic Needles
The SurGuard™2 needle has been developed to meet the market’s requirements and offers you a simple SAFETY solution.
It's simple locking mechanism will reduce needle stick injuries significantly and can easily be activated by using only one hand!
The SurGuard™2 needle’s standard hub eq... -
Product K-Pack™ Surshield™ Needles with passive sharps protection
K-Pack™ Surshield™ needles are hypodermic needles equipped with a passive sharps protection intended to offer needlestick prevention solutions that support therapeutic success. -
Product Surflo™ Winged Infusion Set with/without Needle Protection
Surflo™ Winged Infusion Sets with disposable infusion apparatus, are used for intravenous injection.
Surflo™ Winged Infusion Sets with needle protection (Surshield™) minimize the risk of accidental needle stick. Once activated, the needle is perman... -
Product NEOLUS™ Needles
NEOLUS™ needles are sterile, single-use hypodermic needles, intended to inject fluids into, or withdraw fluids fromparts of the body below the surface of the skin. -
Product Nanopass™ 32.5G Pen Needle
Our Nanopass™ 32.5G Pen Needle for Pen Injector offers a reduced diameter thin-wall needle and unique asymmetric lance bevel. -
Product Nanopass™ 34G Pen Needle
Nanopass™ 34G Needle for Pen Injector features a 22% thinner double-tapered needle aiming at a better flow than comparable 32G designs. -
Product K-Pack™ II Needles
K-Pack™ II Needles are carefully crafted to achieve smoother injections of today and tomorrow’s parenteral medications – targeting on a less painful injection and thus on higher therapeutic adherence and compliance. -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Tapered Needle
PLAJEX™ Ready-to-Fill Polymer Syringes with Tapered Needles offer the better injectability without compromising patient comfort for pharmaceutical manufacturers who need to overcome injectability problems associated with highly viscous concentrated formulations.
(Assessment of the Injection Performa... -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Luer Lock
PLAJEX™ Ready-to-Fill Polymer Syringes with Luer Lock offerthe quality and supply reliability needed to meet your parenteral delivery challenges. -
Product SurGuard™3 Safety Hypodermic Needles
SurGuard™3 Safety Hypodermic Needles featured by Terumo Medical Care Solutions Pharmaceutical Division are equipped with a three-way needlestick protection system and utilize Terumo’s T-Sharp™ technology – offering Terumo quality needles. -
Product PLAJEX™ Ready-to-Fill Polymer Syringe with Staked Needle
PLAJEX™ Ready-to-Fill Polymer Syringes with Staked Needles provide lower interactions with proteins for pharmaceutical manufacturers who need to deliver today’s most challenging biopharmaceuticals.
(Functional Evaluation and Characterization of a Newly Developed Silicone Oil-Free Prefillable Syr...
TERUMO resources (14)
-
News Pharmapack 2025 Track Sponsor Interview: Terumo on the importance of packaging innovation
At Pharmapack 2025 in Paris in January, the conference theatre will once again be hosting insightful discussions across several key topics in the pharmaceutical packaging field. One track, Device and Packaging Innovation will be sponsored by Terumo. -
Video Terumo Medical Care Solutions - CDMO
Access world-class CDMO capabilities.
We provide sterile injectable CDMO from clinical to commercial scale. Drawing on 100 years of expertise, our integrated services include combination product design, formulation development, aseptic fill & finish, device assembly and packaging. -
News Pharmapack 2023: Session interview – Terumo on integrated CDMO solutions
Pharmapack 2023 showcased a number of sessions on the key topics in the industry, from conference sessions to workshops, presented by experts in the field. -
Video Pharmaceutical Solutions Division in video
Discover who we are and how we work. Get a glimpse of our state of the art production. -
News CPHI Frankfurt 2022: Innovator Interview – Terumo
In this series of interviews, we speak to the companies on the CPHI Frankfurt show floor who are driving innovation in pharma for a better healthcare future. We chatted to Marco Chiadò Piat (President) and Anil Busimi (VP Strategy and Marketing) of Terumo Pharmaceutical Solutions about how events such as CPHI can help all companies across the pharmaceutical supply chain. -
Video Keynote: Reusability Simplified: The Journey to Develop a Sustainable, Low-Cost Reusable Autoinjector
Device reusability has gained increasing attention in recent years, owing toits potential to reduce both the costs and environmental impact of drug delivery devices. This has made reusable autoinjectors an appealing and potentially more sustainable option, especially when considering chronic conditions which require frequent dosing. Compared to single use autoinjectors, a reusable component inevitably adds use steps to load, unload and reset the device for each dose. Reusable handsets offer the potential for electromechanical and connectivity features that can improve usability but typically at a significant additional cost and at the risk of off setting many of the sustainability benefits that device reusability can offer. A collaboration between AstraZeneca and Team Consulting set out to explore whether it is possible to realize the cost and sustainability benefits of reuse without compromising on usability. This talk will explore an innovative, mechanical solution for a reusable autoinjector that is designed to meet this goal. -
Video Keynote Address: Global Insight – What Is New in the Packaging & Device Sector
This keynote will provide a comprehensive overview of the latest trends and developments in the packaging and device sector, with a special focus on emerging markets like China and countries in the Southern Hemisphere. The session will also cover crucial updates on regulations and compliance requirements, equipping attendees with the knowledge to navigate the evolving global landscape in this dynamic industry. -
Video Lightning Talk: Ophthalmic Drug Delivery Latest Development
The development of new ophthalmic drop delivery systems represents a crucial challenge when it comes to meeting the growing needs of eye disease treatment. Since the 1950s, these systems have evolved considerably, driven by both technological and regulatory advances. Technological innovation plays a central role in this field, with advances such as preservative-free multi-dose vials that improve tolerance in sensitive patients. Tomorrow, these innovations should translate into eco-designed systems, facilitating personalized and optimized treatments, while also improving patient compliance. In addition, the rise of new technologies such as nanotechnologies, biopolymers and digital devices is paving the way for a new generation of sustained-release systems. -
Video Lightning Talk: Innovation in Production of Packaging Solutions For Sterilization Processes
The production of packaging for sterilization processes, especially considering the Annex 1, requires a robust contamination control strategy and innovative solutions to enhance safety and efficiency. AM Instruments presents essential and innovative elements that define a new standard in the packaging management. This strategy adheres to contamination control protocols and is built on four main pillars: Safety: Establishing and maintaining alert levels to monitor and control contamination risks. Choice of Material: Evaluating materials based on factors such as minimal particle release, resistance, and permeability to ensure optimal performance and safety. Long Life Solution: Developing durable packaging solutions that maintain integrity over extended periods. Efficient Use of Final Product: Implementing practices to reduce operating times and minimize risks associated with high handling and consequent particle release, thereby improving overall efficiency and safety. -
Video Lightning Talk: Designing for an EMERGENCY
Do we understand the true reality of what someone who isn't a Healthcare Professional goes through when they open the packaging and prepare to use a medical device in an emergency? After decades of feeding into the design and development of these devices, I was unfortunately one of those people. If I hadn’t performed the use steps correctly, my little boy would have died. How well suited are packaging designs for emergency use, and how well do they factor in the emotional, psychological, physiological, and cognitive condition someone experiences in an emergency. Do formative and summative studies adequately simulate emergency scenarios when evaluating packaging designs? Let's take a step back, hear from people who have first-hand experience using products such as glucagon pens and EpiPens, and design for emergencies.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






















.png)

%20(1).png)
%20(1).png)
.png)
%20(1).png)













-comp247874.png)





-comp282059.jpg)