TBFA
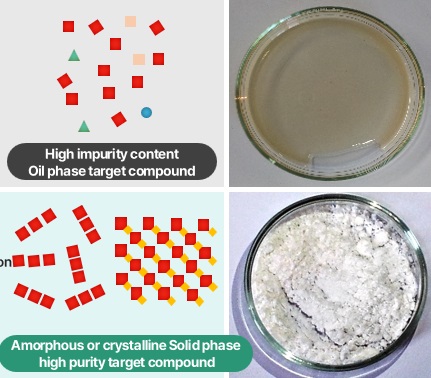
Product Description
MFC Co.,Ltd.

-
KR
-
2022On CPHI since
-
3Certificates
-
50 - 99Employees
Company types
Primary activities
Categories
Specifications
MFC Co.,Ltd.

-
KR
-
2022On CPHI since
-
3Certificates
-
50 - 99Employees
Company types
Primary activities
More Products from MFC Co.,Ltd. (1)
-
Product Synthesis of APIs
APIs: Pitavastatin Calcium Hydrate, Rosuvastatin Calcium, Donepezil, Clopidogrel Bisulfate, Sarpogrelate Hydrochloride, Erdosteine, Pelubiprofen, (S)-Amlodipine Besylate 2.5 Hydrate, (S)-Pantoprazole Sodium Trihydrate
Intermediates of Statins:
MRV-1, ...
MFC Co.,Ltd. resources (1)
-
Video MFC Co.,Ltd
MFC Co., Ltd., established in 2008, is a manufacturer specializing in APIs and key intermediates, which GMP certified from KMFDS. MFC is continuously developing under quality management through thorough quality control and quality assurance system, and technology management that put best efforts in continuous process improvement and new technology development. MFC's direction and aim are the development of new excellent new APIs and intermediates, CMO/CDMO business in accordance with customer requirements, and Incrementally Modified Drugs (IMD) & New Chemical Entity (NCE) using specialized technologies to go forward as a global pharmaceutical company.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


