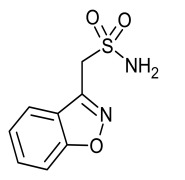
Tauroursodeoxycholic Acid

Product Description
Dipharma

-
IT
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Dipharma

-
IT
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Dipharma (40)
-
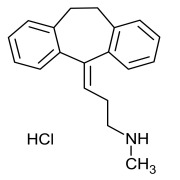
Product Nortriptyline Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Nortriptyline Hydrochloride. It belongs to commercial products category. Therapeutic category: Antidepressant. Contact us for more information. -
Product Pirfenidone
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Pirfenidone. It belongs to new products category. Therapeutic category: Immunosuppressant. Contact us for more information. -
Product Piroxicam
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Piroxicam. It belongs to commercial products category. Therapeutic category: Anti-inflammatory. Contact us for more information. -
Product Rufinamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Rufinamide. It belongs to new products category.
Therapeutic category: Anticonvulsant.
Contact us for more information. -
Product Selegiline Base*
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Selegiline Base*. It belongs to new products category. Therapeutic category: Antidepressant, Antiparkinson. Contact us for more information.
* only for specific markets. See our website -
Product Selegiline Hydrochloride
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Selegiline Hydrochloride. It belongs to commercial products category. Therapeutic category: Antiparkinsonian. Contact us for more information. -
Product Tolbutamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Tolbutamide. It belongs to commercial products category. Therapeutic category: Antidiabetic. Contact us for more information. -
Product Triamterene
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Triamterene. It belongs to commercial products category. Therapeutic category: Diuretic. Contact us for more information. -
Product Varenicline Tartrate
Dipharma manufactures wide range of active pharmaceutical ingredients including Varenicline Tartrate, a smoking-cessation product, which is nitrosamine free due to the dedicated process developed. Contact us for more information.
-
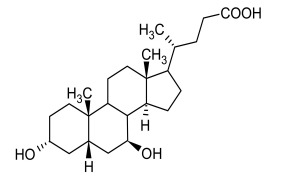
Product Ursodeoxycholic Acid
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Ursodeoxycholic Acid. It belongs to commercial products category. Therapeutic category: Cholelitholytic Agent. Contact us for more information. -
Product Vortioxetine HBr
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Vortioxetine HBr. It belongs to new products category. Therapeutic category: Antidepressant. Contact us for more information. -
Product Zonisamide
Dipharma manufactures wide range of active pharmaceutical ingredients which includes Zonisamide. It belongs to commercial products category. Therapeutic category: Anticonvulsant. Contact us for more information.
Dipharma resources (16)
-
News Successful FDA Inspection at Dipharma Facility in Kalamazoo, MI, USA
Dipharma Inc. has been inspected by the US FDA and has received the EIR, satisfactorily closing the inspection process. The Company expands its range of CGMP services to include small-scale commercial APIs. -
Video Development and Manufacturing of New APIs: Leveraging Experience, Technological Innovation, and Present Vision to Future Success.
In today’s globalized economy, international integration plays a crucial role in the development of new molecules. This integration encompasses various aspects, including the transfer of production activities and the utilization of past experiences. Risk and Innovation: The development of APIs (active pharmaceutical ingredients) utilizes innovative process techniques and potentially hazardous chemical reactions. Leveraging past experiences is essential to mitigate risks and optimize production. Solid-State Development: A deep understanding of solid-state properties is critical for API manufacturers. Solid-state characterization ensures product stability, bioavailability, and manufacturability. -
News Dipharma expands its Italian R&D Center to increase its analytical services
The Company strengthens its analytical R&D capabilities, to better support its customers operating in the CDMO and Generics market.
-
Video The Dipharma Group - Experience you can trust
The Dipharma Group is a global Contract Development and Manufacturing Organization (CDMO) and a leading manufacturer of Active Pharmaceutical Ingredients (APIs), New Chemical Entities (NCE), and advanced Intermediates for Generic and Contract Manufacturing markets, offering APIs with DMFs registered worldwide.
-
News Dipharma completes second phase of CGMP expansion at its Kalamazoo site
The Company continues to strengthen its capabilities in the North American market -
Video Dipharma's CGMP pilot plant in Mereto di Tomba, Italy
The CGMP pilot plant located in our production site of Mereto (Italy) is equipped with cutting-edge technologies, providing powerful tools to supply from early drug development up to the market. The pilot plant consists of two production lines, each equipped with 2 stainless steel and 2 glass-lined reactors and can produce batches ranging from 5 to 50 kilograms.
Our state-of-the-art equipment, combined with demonstrated expertise and flexibility, makes Dipharma the optimal choice to support your projects.
EXPERIENCE YOU CAN TRUST. -
News Dipharma receives GMP certification from Brazilian ANVISA
The Baranzate site (Italy) is the first Dipharma manufacturing facilityto receive Anvisa certification -
Brochure Dipharma's Catalogue APIs
Dipharma develops proprietary chemical processes and alternative solutions to support the generic API industry.Our customers can rely on a true turnkey service, where commercially competitive products are promptly conceived and manufactured in full compliance with third-party intellectual property rights.
Along with quality, confidentiality, safety and meeting deadlines, we ensure a well-established and reliable supply chain for your peace of mind.
Our current product portfolio comprises over 40 high quality APIs. -
News Regulatory authorization for the new line at cGMP pilot plant
Thanks to the approval by the Italian Medicines Agency (AIFA),the Company increases its efficiency and is able to face the growing demand for its services -
Brochure Dipharma's Exclusive Synthesis
Achieve your goals more easily, safely and rapidly.The ability to develop innovative synthetic processes, combined with proven know-how in the handling of hazardous chemicals and reactions, makes Dipharma your optimal choice for Exclusive Synthesis services from discovery to the commercial stage.We provide you with tailor-made development and manufacturing of new and complex molecules, all the required analytical and regulatory assistance, as well as support in pre-formulation activities. -
News Dipharma receives EcoVadis Silver Medal
EcoVadis has rated Dipharma Francis’s performance among the top 25% of all organizations surveyed. -
News Merger of Kalexsyn Inc. into Dipharma Inc.
The merger represents the last step of Kalexsyn’s integration into the Dipharma Group -
News Kalexsyn appoints Brian Eklov as new CEO
Kalexsyn Inc. announced that its Board of Directors appointed Brian Eklov to succeed Robert Gadwood, who is retiring, as Chief Executive Officer and Board member. -
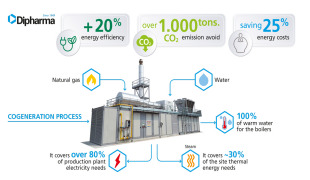
News Dipharma installs a new trigeneration unit
The new plant brings significant positive impact on the environment and sustainable development -
News Dipharma’s high-performance cogeneration unit
Received 468 White Certificates -
News Dipharma builds cGMP manufacturing suite at US subsidiary
Italy’s Dipharma Francis has completed construction of a new cGMP manufacturing suite at its US subsidiary, Kalexsyn, strengthening its service offering and ensuring continuity across the product lifecycle from preclinical to commercialisation , the contract research and development organization announced Monday.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





-file151657.png)