Tamnic

Product Description
Scott-Edil Pharmacia Ltd

-
IN
-
2019On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Scott-Edil Pharmacia Ltd

-
IN
-
2019On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Scott-Edil Pharmacia Ltd (49)
-
Product Scort 40mg INJ
Triamcilonone Acetonide 40 mg -
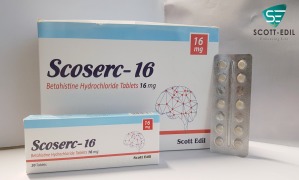
Product Scoserc 16
Betahistine Hydrochloride Tablets 16mg
-
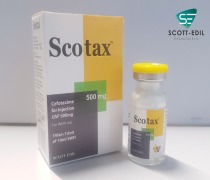
Product Scotax 500mg
Cefotaxime for injection USP 500mg
-
Product Serdon
Dexamethasone & Chlorpheniramine Maleate Syrup
Antihistamine Anti-Inflammatory
-
Product Spascot 40mg/2ml INJ
Drotaverine Hydrochloride Injection
-
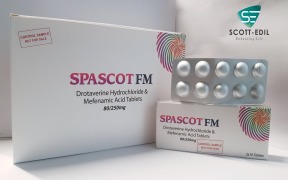
Product Spascot-FM
Drotaverine & Mefenamic Acid -
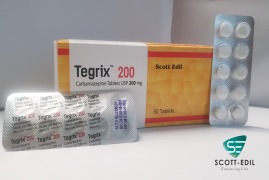
Product Tegrix 200mg
Carbamazepine Tablets 200mg
-
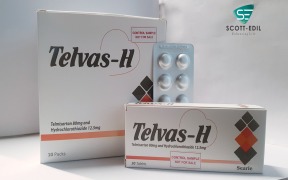
Product Telvas-H
Telmisartan 80mg & Hydrochlorothiazide 12.5mg
-
Product Tenlip
Teneliglipton Yablets 20mg
-
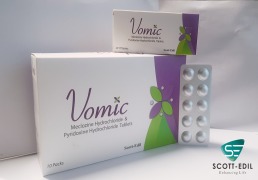
Product Vomic
Meclozine Hydrochloride & Pyridoxine Hydrochloride Tablets
-
Product X-FOR
Amlodipine & Valsartan Tablets
-
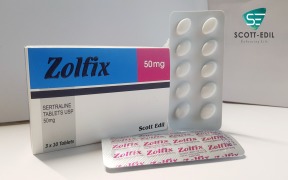
Product Zolfix 50mg
Sertraline Tablets 50mg
Scott-Edil Pharmacia Ltd resources (1)
-
Brochure SCOTT EDIL PRODUCT CATALOGUE
We are one of the leading pharmaceutical manufacturers in India with a portfolio of over 700 drug formulations for various therapeutic segments including antibiotics, analgesics, anti-hypertensives, anti-diabetics, and vitamin supplements.
Our company, WHO-GMP ISO 9001:2015 certified, has three world-class units in Baddi, Himachal Pradesh, and one in Chandigarh, with manufacturing facilities for injections (dry/liquid), ophthalmic solutions, oral liquids, dry syrups, tablets, capsules, gels, hand sanitizer , dietary protein and food supplements under stringent quality-control measures.
The company has registered various trademark certificates and has International dossiers and market authorizations with a presence in more than 60+ countries. With valuable grassroots experience in the field of pharmaceuticals, the company has been able to capitalize on its expertise by expanding into multiple products under its brand—Searle Interphar.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance