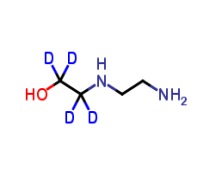
Sterile Sodium Hyaluronate (Hyaluronic Acid)
Product Description
Shandong Topscience Biotech Co. Ltd.

-
CN
-
2015On CPHI since
-
5Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
Shandong Topscience Biotech Co. Ltd.

-
CN
-
2015On CPHI since
-
5Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Shandong Topscience Biotech Co. Ltd. (9)
-
Product Sodium Hyaluronate Pharmaceutical Grade
Pharmaceutical grade sodium hyaluronate which can be used as an API or excipient for drugs and medical devices in ophthalmic preparations, intra-articular injections, anti-adhesive preparations, topical preparations for wound healing and soft tissue filler, etc.
• Written Confirmation for Active ... -
Product Eye drop Grade Sodium Hyaluronate (Hyaluronic Acid )
Eye-drop Grade Sodium Hyaluronate has GMP certification in China and the status of API registration status is A. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
• M... -
Product Injection Grade Sodium Hyaluronate (Hyaluronic Acid)
Injection Grade Sodium Hyaluronate has GMP certification in China and the status of API registration status is A. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
· Microbial ... -
Product Excipient Grade Sodium Hyaluronate (Hyaluronic Acid)
Excipient Grade Sodium Hyaluronate has the registration of pharmaceutical excipients and with high stability. It has the advantages of ultra-low impurity level, ultra-low endotoxin level, ultra-low metal impurity residue and ultra-low microbial pollution.
• Microbia... -
Product Medical Device Grade Chondroitin Sulfate Sodium
Medical Device Grade Chondroitin Sulfate Sodium extracted from shark bone as raw material, fish source is more similar to human DNA and easily absorbed by the human body. And Enzymolysis by special enzymes, is harmless to the human body, and will enzymatic hydrolysis of excess proteins. It can be pur... -
Product Sodium Hyaluronate Food Grade
Sodium Hyaluronate Food Grade is produced by Microbial Fermentation and can promoting health of facial skin, antioxidation, increasing bone mineral density, improving joint function and gastrointestinal function and protecting gastric mucosa.
Mainly in Beverage, jelly, milk products,c... -
Product Sodium Hyaluronate Medical Device Grade
Sodium Hyaluronate Medical Device Grade
Sodium Hyaluronate Medical Device Grade is a kind of Molecular Weight Sodium Hyaluronate Topical Grade 1.2 ~ 3.6 m3/kg. It suitable for medical device of external application. And not suitable for parenteral administration, dermal fillers and not to be used... -
Product Sodium Hyaluronate Medical Injection Grade
Sodium Hyaluronate Medical Injection Grade
Sodium Hyaluronate Medical Injection Grade is a kind of Molecular Weight Sodium Hyaluronate Topical Grade 1.2 ~ 3.2 m3/kg. It Suitable for medical device of parenteral administration and dermal fillers. And not to be used as an API. -
Product Sodium Hyaluronate Topical Grade
Sodium Hyaluronate Topical Grade
Sodium Hyaluronate Topical Grade is a kind of Molecular Weight Sodium Hyaluronate Topical Grade 0.047 ~ 3.6 m3/kg. It suitable for medical device of external application. And not suitable for parenteral administration, dermal fillers and not to be used as an API. ...
Shandong Topscience Biotech Co. Ltd. resources (4)
-
News in-cosmetics global 2024
The 2024 European Cosmetics and Personal Care Ingredients Exhibition (in-cosmetics global 2024) concluded successfully at Paris Expo Porte de Versailles in France. Topscience, as a biotechnology innovative enterprise focusing on the research, production, sales, and service of bioactive substances with core technologies in microbial fermentation and aseptic raw material production, showcased a variety of new products at this exhibition. Among them were products such as Full-spectrum Sodium Hyaluronate and Zinc Hydrolyzed Hyaluronate, attracting considerable attention. -
Brochure Introduction of Topscience Biotech
Topscience Biotech was founded in 2005 and is a biotechnological innovative enterprise focusing on the R&D, production, sales, and service of bioactive with microbial fermentation technology and aseptic raw material production technology as the core. Topscience’s main products include biochemical APIs, tissue engineering raw materials, functional skincare ingredients, novel food raw materials, etc. The main product sodium hyaluronate series, which can be applied to high-end pharmaceutical orthopedics, ophthalmology, advanced medical cosmetology dermal fillers, etc., to provide excellent quality and safety compliance raw material support for global partners to carry out differentiated competition.
Topscience has 8 GMP-compliant production workshops and GLP-compliant laboratories. Also, Topscience Biotech has established a sound quality system in accordance with international standards such as ICH and GMP, and has passed on-site inspections by domes... -
News CPHI Barcelona
Topscience Biotech sincerely invites you to join us! #81B39
CPHI Barcelona
24 - 26 October 2023
Fira Barcelona Gran Via, Spain
-
News Unite Globally | Topscience Successfully Concludes CPHI North America
5月7日至5月9日,美国制药行业顶级展会——世界制药原料北美展(CPHI North America)在费城正式拉开帷幕,本届展会吸引了来自全球360余家展商、5000余名专业观众,展会涵盖了医药原料、合同定制、药品研发、生产、制药机械、包装设备等全产业链。
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


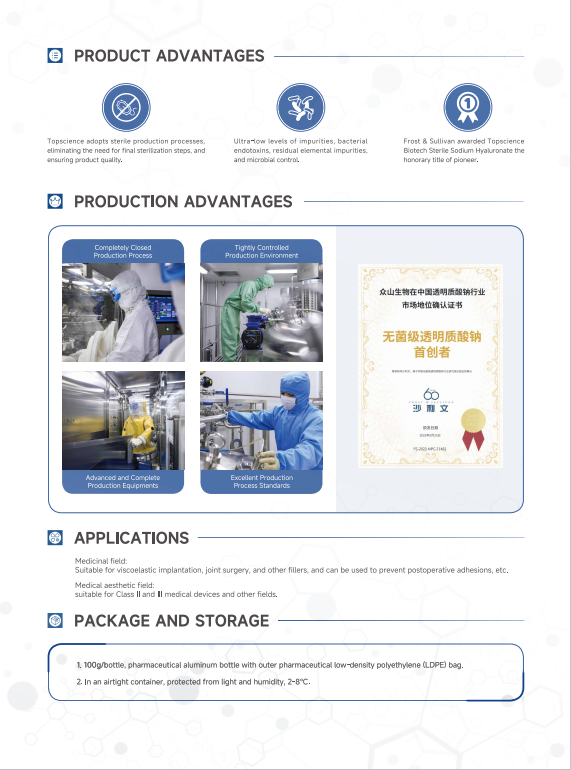






















































.jpg)