Solid-State Characterization
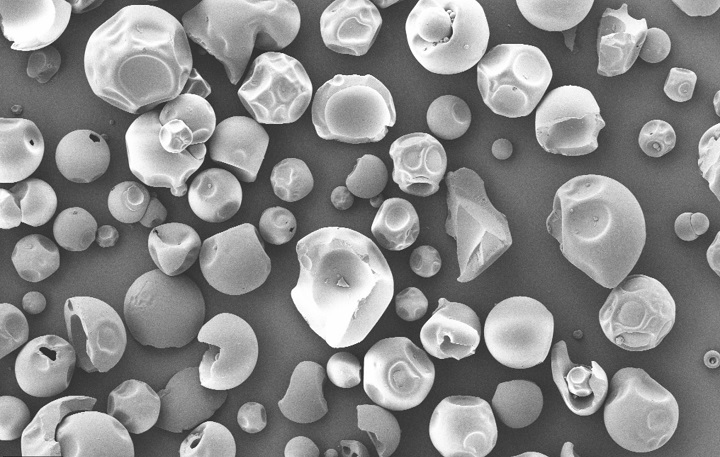
Product Description
Particle Analytical

-
DK
-
2024On CPHI since
-
1 - 24Employees
Company types
Categories
Specifications
Particle Analytical

-
DK
-
2024On CPHI since
-
1 - 24Employees
Company types
More Products from Particle Analytical (2)
-
Product Bioequivalance study by raman microscopy (MDRS)
At Particle Analytical, our expertise in bioequivalence studies using Raman microscopy (Morphologically Directed Raman Spectroscopy, MDRS) allows us to deliver precise and detailed analysis of pharmaceutical products. Raman microscopy is a powerful tool for identifying and characterizing the different comp... -
Product Solid State Screening
Solid-state screening is a crucial process in pharmaceutical development, aimed at identifying and characterizing the most stable and suitable solid forms of a drug substance. At Particle Analytical, our solid-state screening services provide comprehensive analysis to determine the optimal polymorph, salt,...
Particle Analytical resources (1)
-
Technical Data Particle Analytical Technical Sheet - laboratory equipment
Explore the Particle Analytical company presentation to learn more about our expertise in particle size and crystal analysis. With over 20 years of experience, we are well-equipped to support you through all phases of drug discovery, development, and manufacturing. We continually enhance our capabilities, and our latest advancement is GMP certification for Raman microscopy - please note, that this is not updated in the Technical Sheet yet.
Additionally, we offer polymorph screening and assistance with bioequivalence studies.
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance


