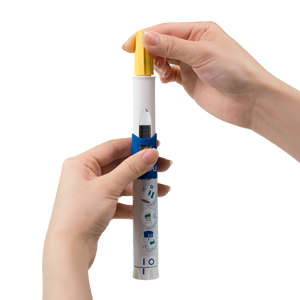
SDI MIX®+NIT® Autoinjector
Product Description
SHL Medical

-
CH
-
2020On CPHI since
-
2Certificates
-
5000+Employees
Company types
Categories
Specifications
SHL Medical

-
CH
-
2020On CPHI since
-
2Certificates
-
5000+Employees
Company types
More Products from SHL Medical (9)
-
Product Maggie® 5.0 Autoinjector
Maggie® 5.0 is an innovative, large-volume, cartridge-based autoinjector built with SHL’s market-proven Needle Isolation Technology (NIT®). A two-step autoinjector, it was developed with a focus on opening a new pathway for handheld devices to accommodate a wider variety of formulation characteristics, suc... -
Product Molly® Connected Cap Autoinjector
The Molly® Connected Cap is a compact, retrofittable autoinjector add-on that records and transmits data on patients’ use of the device. Upon cap removal from the Molly device, the Connected Cap becomes active, allowing timestamped data to be relayed either through a smartphone app or smart data transmissi... -
Product Elexy™ autoinjector
Elexy™ is a versatile, electromechanical autoinjector that features a reusable power unit compatible with drug cassettes containing a pre-filled syringe or a cartridge. Its versatility enables a wider choice of intended injection profiles, including drug volume, drug viscosity, and injection speed. In ... -
Product Needle Isolation Technology (NIT®)
NIT® is a safety solution that eliminates the need for manual needle attachment in cartridge-based injections. It enables cartridges to be built into autoinjectors, supporting treatments beyond the 2.25 mL fill range and lyophilized drugs requiring dual-chamber containers.
Products built with NIT ... -
Product Autoinjector development services
SHL Medical’s commitment to innovation has driven our leadership in the drug delivery industry. We offer vertically integrated development services to ensure the production of our self-injection systems are delivered with unparalleled speed, quality, and reliability.
-
Product VSDI(R)+NIT(R) Autoinjector
VSDI+NIT is a cartridge-based autoinjector built with SHL's Needle Isolation Technology (NIT(R)). NIT eliminates the need for patients to manually attach the needle before each injection. With cartridge compatibility ranging from 1.5 mL to 3.0 mL, this autoinjector's dosing variations can be customized to ... -
Product Molly® Modular Platform Autoinjector
Molly® is a single-dose autoinjector technology developed to help pharma and biotech companies reduce initial investments and expedite development timelines. Products using Molly autoinjectors have been approved and launched in 14 molecular entities covering at least 25 clinical indications in a variet... -
Product Molly® 2.25 Modular Platform Autoinjector
Molly® 2.25 is a large-volume variant of the Molly autoinjector to accommodate larger-volume biologics. Featuring the same easy-to-use, 2-step operation, it inherited the modular platform development model to support high-volume manufacturing, faster development, and flexible design for unique branding nee... -
Product Maggie® Autoinjector
Maggie is a cartridge-based autoinjector that accommodates fill volumes of up to 3.0 mL. Built with SHL’s market-proven Needle Isolation Technology (NIT®), this 2-step autoinjector uses a pre-attached and sterile needle housed within the device cap. The needle automatically attaches to the cartridge upon c...
SHL Medical resources (47)
-
News SHL Medical releases the 2023 sustainability report
SHL Medical is pleased to announce the release of its 2023 sustainability report, which details the company’s ongoing efforts and accomplishments in sustainability and innovation. -
Video Molly® Modular Platform Autoinjector
SHL embarked on a journey of transformation with the Molly® autoinjector. After successfully supporting the development of more than a dozen combination products globally, we decide to take the innovation up a notch.
THIS is the next generation of Molly and Molly 2.25. Built upon a modular platform technology, Molly now offers flexibility beyond a conventional platform model that spans across the development processes.
Get an in-depth understanding of how Molly works to address the varying requirements of the industry and learn more about SHL's offerings at our website.
-
News SHL Medical and AARDEX Group forge strategic alliance to interface medication adherence software with connected self-injection solutions
SHL Medical and AARDEX Group announced their strategic partnership aimed at delivering an end-to-end solution for pharma customers seeking to demonstrate patient adherence within clinical trials. -
Video Maggie®: Rethink Your Self-Injection
The constant evolution of drug formulations across various disease areas opens a wider avenue for subcutaneous drug delivery. SHL's Maggie® is a cartridge-based autoinjector that requires just two steps to operate. Built with the market-proven Needle Isolation Technology (NIT®), the device can accommodate varying formulations with volumes up to 3.0 mL. Following that, SHL is excited to introduce Maggie 5.0 mL − the newest member of our NIT device family, developed to potentially accommodate injection volumes previously not considered by traditional autoinjectors.
-
News Aptar Digital Health and SHL Medical enter strategic partnership to optimize the patient experience with self-injectable therapies
SHL Medical and AARDEX Group announced their strategic partnership aimed at delivering an end-to-end solution for pharma customers seeking to demonstrate patient adherence within clinical trials. -
Video Make Every Injection Count with Molly® Connected Cap
Ready to embark on a journey where connectivity meets autoinjectors? Dive into the world of SHL Medical’s Molly® Connected Cap and experience the future of healthcare.
Driven by its experience with commercializing over three dozen autoinjectors worldwide, including 17 combination product projects from the market-proven Molly modular platform technology, SHL Medical developed the Molly Connected Cap autoinjector – an agile solution that supports remote adherence monitoring for personalized care. -
News Committed to a sustainable future: SHL Medical’s Sustainability Report 2022
SHL Medical announced today the release of its Sustainability Report for 2022. The report provides a comprehensive overview of the company’s efforts to promote sustainability and outlines its commitment to a sustainable future. -
Video Redefining possibilities with Elexy™
Elexy™ is a versatile, electromechanical autoinjector that features a reusable power unit compatible with drug cassettes containing a pre-filled syringe or a cartridge. Its versatility enables a wider choice of intended injection profiles, including drug volume, drug viscosity, and injection speed. In addition, Elexy features connectivity with cellular and Bluetooth® options to collect comprehensive injection-related data. -
News SHL Medical acquires 100% of the shares in Swiss company LCA Automation
SHL Medical announces the acquisition of LCA Automation AG, a Swiss innovative automation solutions provider. The acquisition is SHL Medical’s response to the growing market demand for drug delivery solutions and will support its manufacturing operations globally, especially the upcoming Swiss manufacturing site in Zug. -
Brochure Your drug delivery partner with proven track record
From design to mass production, SHL Medical's end-to-end device development infrastructure ensures the lasting value of self-injection systems that we design and develop with our customers. Our comprehensive in-house manufacturing capabilities and expertise include every core competency and value-added services across the product lifecycle. 35 years of pioneering the drug delivery industryOver 50 combination products launchedTop 25 bio/pharmaceutical companies in partnership Over 120M SHL devices reached patients worldwide -
News SHL Medical and MoonLake Immunotherapeutics collaborate to develop sonelokimab autoinjector
SHL Medical, a world-leading provider of advanced drug delivery solutions, announced that it has signed a collaboration agreement with MoonLake Immunotherapeutics, a clinical-stage biotechnology company focused on creating next-level therapies for inflammatory diseases, to develop an autoinjector for clinical and potential commercial supply of MoonLake’s Nanobody® sonelokimab based on SHL Medical’s Molly® autoinjector technology. -
Whitepaper Molly Autoinjector White Paper
A retrospective analysis on how the new generation of Molly autoinjectors have incorporated a greater level of flexibility in its design and development model. -
News SHL Medical further strengthens vertical capabilities with the acquisition of US manufacturer Superior Tooling Inc.
Continuing its ambitious growth journey, SHL Medical has acquired Superior Tooling Inc., a US-based manufacturing company specializing in plastic injection molds. The integration of Superior Tooling will strengthen the SHL’s inhouse manufacturing capabilities, particularly for its upcoming US manufacturing site in North Charleston, SC, scheduled to begin operations in mid-2024. -
Brochure Autoinjectors From Planning to Launch
Explore the complete journey of autoinjectors, from initial planning to successful launch, with insights into design, development, and market readiness. -
News SHL Medical acquires Swiss toolmaking company SMC Mould Innovation
SHL Medical, a leading provider of drug delivery solutions worldwide, furthers its expansion strategy by acquiring SMC Mould Innovation, a Swiss manufacturer specialising in high-performance injection molding tools, based in Hallau. -
Brochure SHL Medical Sustainability Report 2023
SHL Medical's 2023 sustainability report details the company’s ongoing efforts and accomplishments in sustainability and innovation. The report emphasizes the company’s goals, initiatives, and progress towards three sustainability goals it has set.
Driving patient health and independenceReducing ecological footprintEnsuring responsible business practices across supply chain, employees, and its communities. -
News ten23 health® and SHL Medical announce a strategic partnership agreement for integrated services
SHL Medical, a world-leading provider of drug delivery devices, and ten23 health®, a leading Swiss Contract Development and Manufacturing Organization (CDMO) for sterile drugs, announced strategic partnership to provide a streamlined offering for pharmaceutical and biotech companies that require sterile drug/device combination products. -
Video SHL Medical: Innovation in Motion
Innovation in Motion highlights SHL Medical's dynamic journey of global expansion and innovation. In this video, we showcased how SHL has established a strong presence across continents, strategically expanding our facilities to meet the growing demands of autoinjector industry. With state-of-the-art manufacturing sites, research and development centers, and logistics network worldwide, SHL Medical is committed to bringing innovative self-injection solutions to patients everywhere.
From the Americas to Asia to Europe, SHL's global network allows us to deliver consistent quality and innovation, no matter where we are. Each location plays a vital role in our mission to improve patient care, offering local expertise while contributing to our global vision.
-
News SHL Medical partners with SteriPack Contract Manufacturing to set up final assembly service
SHL Medical, a world-leading solutions provider of advanced drug delivery systems and SteriPack Group, renowned global supplier of secondary packing and final assembly services to the pharmaceutical industry, enter a non-exclusive strategic partnership. -
Video How SLIM enhances assembly
Meet SLIM (SMART-Line Integrated Measurement) – our state-of-the-art technology designed to replicate critical assembly steps in a lab environment. With the ability to process both sub-assembly and final assembly on the same equipment, SLIM supports multiple device variations, making it ideal for development, clinical, and low-volume commercial production. It’s a perfect example of how we integrate advanced technology to enhance flexibility and efficiency in our development processes.
-
News SHL Medical and FUJIFILM Diosynth Biotechnologies enter strategic partnership agreement
SHL Medical (SHL), a world-leading solutions provider of advanced drug delivery systems enters a non-exclusive strategic partnership agreement with FUJIFILM Diosynth Biotechnologies, a world-leading contract development and manufacturing organization (CDMO) for biologics, vaccines, advanced therapies and oncolytic viruses to help meet growing market demand for autoinjector medicines. -
Video Delivering quality without compromise with SMART
As labor costs and demand for autoinjectors rise, a fully automated assembly machine with the flexibility to produce smaller batches has quickly become the much-needed solution. SHL’s SMART is pioneering the path to address this need with its ability to combine the flexibility of manual processes with the efficiency and reliability of automated systems. Its modular design combines physical components with digital controls for versatile assembly and efficient project changeovers. -
News SHL Medical achieves EcoVadis® Silver Medal and sets ambitious science-based climate targets
SHL Medical, a world-leading provider of advanced drug delivery solutions, is a proud recipient of the prestigious EcoVadis® Silver Medal for its sustainability performance. The company received further recognition from the Science Based Targets initiative (SBTi) for its near-term emissions targets. -
Video Fully Automated Assembly Machine
As an industry leader not only in device design and development but also in product industrialization, SHL Medical's automation systems department develops advanced assembly machines, allowing us to offer fully automated solutions for rigorous testing and production needs. SHL's in-house fully automated assembly machine offers the reliability to produce high-volume productions with identical quality from the first assembled unit until the last unit. -
News SHL Medical and Lifecore Biomedical enter co-marketing partnership agreement
The partnership between SHL Medical and Lifecore Biomedical will allow the companies to exchange knowledge and experience in their respective fields and provide their customers unparalleled guidance in CDMO services and best-in-class options for drug delivery device development. -
Video Final assembly, labeling, and packaging
SHL Medical offers final assembly, labeling, and packaging services for drug delivery devices. We have a modern facility with expanding competencies – an industry first-in-class site that provides final assembly equipment qualification, process development, design transfer, and turnkey final assembly services. The facility is further equipped with device labeling, carton packaging, and serialization capabilities, providing an end-to-end solution for final combination products.
In addition, SHL Medical offers stability and transportation studies. By offering this full suite of development solutions, we help our clients optimize timelines to meet both clinical and global market needs. -
News SHL Medical launches new webpage for Molly Connected Cap autoinjector
SHL Medical, a leader and front-runner in advanced technologies for autoinjectors and specialty drug delivery systems, announced the launch of a new webpage for its Molly® Connected Cap, a smart, retrofittable add-on for the market-proven Molly modular platform autoinjector. -
Video The Makings of an Autoinjector
SHL’s commitment to our partners as well as the patients they serve stems from a culture deeply rooted in our corporate vision − that of delivering quality and leadership through innovative solutions that empower people.
-
News SHL Medical announces strategic alliance program for autoinjector services
SHL Medical is forging alliances with a selection of solutions providers along the drug delivery value chain, including with primary container providers, CMOs/CDMOs, and various service providers and suppliers of primary and secondary packaging, to further strengthen its vertically integrated business model. -
Podcast Podcast: Leveraging data science for sustainable autoinjector development
Sustainability is becoming increasingly important in the pharma and biopharma industries, with many companies working to incorporate more eco-friendly materials, minimise waste and improve social impact. One such company is SHL Medical, a producer of advanced delivery devices, who are utilising data science to advance their sustainability goals. In this podcast, we discuss the relationship between data and sustainability and hear from SHL how their data-driven approach has produced ‘win-win scenarios’ for the company, its customers and the planet. -
News Webinar: Digitalization of autoinjector manufacturing
In this webinar, the speakers will help you understand the intricacies of digitalization in autoinjector manufacturing, reporting on the key learnings from implementing end-to-end digital processes – from product and process development down to the shop floor and machine monitory systems.
-
Technical Data Molly® Connected Cap Autoinjector Datasheet
The Molly® Connected Cap is a compact, retrofittable autoinjector add-on that records and transmits data on patients’ use of the device. Upon removal from the Molly® device, the Connected Cap becomes active, allowing timestamped data to be relayed through a smart data transmission hub and to the cloud. -
News CPHI Podcast Series: Leveraging data science for sustainable autoinjector development
Environmental impact is an area of increasing focus for the pharma and biopharma industries, with companies like Merck, Pfizer and GlaxoSmithKline setting ambitious targets for carbon neutrality and laying plans for waste reduction. -
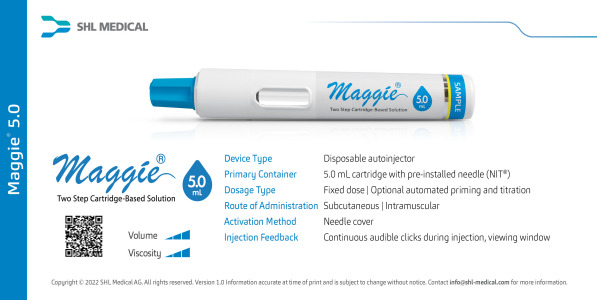
Technical Data Maggie® 5.0 Autoinjector Datasheet
Maggie 5.0 mL is the large-volume variation of the Maggie cartridge-based autoinjector, built with SHL’s market-proven Needle Isolation Technology (NIT®). Maggie® accommodates a wider variety of formulation characteristics and volumes that are not possible with pre-filled syringe-based autoinjectors. -
Sponsored Content SHL partner Innovation Zed announces plans to launch InsulCheck DOSE
SHL Medical and Innovation Zed have announced plans to launch InsulCheck® DOSE in 2022. -
Technical Data Maggie® Autoinjector Datasheet
Maggie® is a cartridge-based autoinjector technology built with our market-proven Needle Isolation Technology (NIT®). Maggie® accommodates a wider variety of formulation characteristics and volumes that are not possible with pre-filled syringe-based autoinjectors. -
News SHL Medical publishes its Molly® modular platform technology white paper
This white paper is a retrospective analysis on how the new generation of Molly autoinjectors have incorporated a greater level of flexibility in its design and development model. -
Technical Data Molly® 2.25 Modular Platform Autoinjector Datasheet
Molly® 2.25 is a large-volume variant of the Molly autoinjector to accommodate larger-volume biologics. Featuring the same easy-to-use, 2-step operation, it inherited the modular platform development model to support high-volume manufacturing, faster development, and flexible design for unique branding needs. -
News SHL Medical launches dynamic webpage for Molly autoinjector technology
SHL Medical announced the launch of its Molly autoinjector webpage. -
Technical Data Molly® Modular Platform Autoinjector Datasheet
Molly® is a single-dose autoinjector technology developed to help pharma and biotech companies reduce initial investments and expedite development timelines. Built on a modular platform technology, Molly® is designed to meet the evolving requirements of future autoinjectors, such as introducing unique designs for branding and user requirements, as well as high-volume manufacturing.
The Molly autoinjector is designed with an ergonomic body and a flange-shaped anti-roll cap for an easier handling experience.
-
News Beyond the platform – applying modularity to autoinjector development
SHL’s Molly® and Molly® 2.25 mL autoinjectors are built upon a proven modular platform technology to support shorter development timelines and increased flexibility in device design and production. -
Technical Data Elexy™ for Cartridge Autoinjector Datasheet
Elexy™ is a versatile, electromechanical autoinjector with a reusable power unit compatible with drug cassettes containing a pre-filled syringe or cartridge.
Elexy aims to provide a best-in-class user experience and a wide range of injection profiles. Comprised of a reusable power unit and disposable drug cassettes, the Elexy system is compatible with both pre-filled syringe (PFS) and cartridge primary containers and, capable of delivering up to 5.0 mL in a single injection. -
Technical Data Elexy™ for PFS Autoinjector Datasheet
Elexy™ is a versatile, electromechanical autoinjector with a reusable power unit compatible with drug cassettes containing a pre-filled syringe or cartridge.
Elexy aims to provide a best-in-class user experience and a wide range of injection profiles. Comprised of a reusable power unit and disposable drug cassettes, the Elexy system is compatible with both pre-filled syringe (PFS) and cartridge primary containers and, capable of delivering up to 5.0 mL in a single injection. -
Video Fully Automated Assembly Machine
As an industry leader not only in device design and development but also in product industrialization, SHL Medical's automation systems department develops advanced assembly machines, allowing us to offer fully automated solutions for rigorous testing and production needs. SHL's in-house fully automated assembly machine offers the reliability to produce high-volume productions with identical quality from the first assembled unit until the last unit.
-
Video A Drug-Agnostic Electromechanical Device Platform to Facilitate the Flexible Development of Combination Products
In this presentation, we introduce our most recent development – a versatile, reusable, and connected electromechanical solution that upholds the concept of drug agnosticism, platform flexibility, ease-of-use, as well as device programmability. By leveraging technological application expertise, we demonstrate how an electromechanical, versatile device platform can flexibly host different primary containers and formulations. Here, we also demonstrate how adding programmable firmware into the device circumvents formulation uncertainties across the pre-clinical, clinical, and commercial development of the drug counterpart. Finally, we show how the conserved element of the device results into an autoinjector solution that flexibly addresses various primary containers, therapy areas, regional healthcare landscapes, as well as patient groups. We also discuss an optional function for tracking injection-related data, opening pathways to gather detailed insights into the patient experience, and ultimately opening possibilities to connect such device usage data with other digital health solutions. -
Webinar The Integration of Digital With Mechanical: Explorations of Connectivity and Data Generation via Our Innovation Partnership Program for the Molly Autoinjector
Abstract: Digital health solutions create myriad possibilities in terms of enabling “beyond the pill” health outcomes. However, the path to streamlining a strategic approach between pharma and its development partners toward creating smart combination products remains a challenge. With a general consensus that developers must accurately determine in advance what users want, many companies wait upon the success of early players as a determinant to foray into digital health. This philosophy, however, disregards the heterogeneity of combination products in development along with the diversity of clinical or patient-reported outcomes measures. Here, we present a dissection of SHL’s Innovation Partnership program along with our connected device offering – both of which allows us to flexibly collaborate with any pharmaceutical partner in the development of connected therapeutics.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

































































-file155071.png)

-file145532.png)