RheaLyo™ GMP-Flex

Product Description
RheaVita

-
BE
-
2023On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
Categories
RheaVita

-
BE
-
2023On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
More Products from RheaVita (4)
-
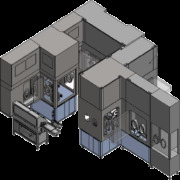
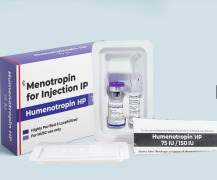
Product RheaLyo GMP-Flex Freeze-Dryers
RheaVita has turned pharmaceutical freeze-drying from a multi-day process into a fast, flexible, and agile process while guaranteeing quality at the individual product level. This is critical to delivering new therapeutics all over the world and making medicines more readily available. The GMP Continuous F... -
Product RheaLyo Mono- Single Vial Unit Freeze-Dryer
RheaVita’s Single Vial Unit (SVU) is based on continuous freeze-drying technology and is specifically designed for R&D purposes to facilitate (bio)pharmaceutical product development. Perfectly suited for very fast product and process development, with minimal material consumption. SVU data as baseline for ... -
Product RheaLyo Mono Freeze-Dryer
The Single Vial Continuous Freeze-Drying System is specifically designed for R&D production based upon the RheaVita Continuous Freeze-Drying Technology. The equipment is perfectly suited for very fast formulation and process development and optimization using a very limited amounts of product by mimick... -
Product Services offered
1. Process development service: RheaVita has built deep knowledge to dry a broad range of biological products. We can design a spin freeze-drying recipe for your compounds in just a few days. 2. Formulation advice: RheaVita’s years of expertise and knowledge of biopharmaceutical stability and degradation a...
RheaVita resources (5)
-
Brochure Continuous and Controlled Freeze-Drying
Improve the quality of your biologicals and streamline your manufacturing with our controlled, continuous freeze-drying technology. -
Brochure RheaLyo Mono Freeze-Dryer (Single Vial Unit)
RheaVita’s Single Vial Unit (SVU) is based on continuous freeze-drying technology and is specifically designed for R&D purposes to facilitate (bio)pharmaceutica -
Brochure Fast, Flexible Freeze-Drying Process for GMP Production
Innovative manufacturing technologies are necessary to make current and future drug products available for each patient, worldwide. -
Brochure White Paper: Continuous freeze-drying paradigm shift
Continuous manufacturing is a valuable investment for companies looking to stay competitive and meet customer demands. -
Whitepaper Continuous Freeze-drying
How continuous freeze-drying fits in the paradigm shift from batch to continuous manufacturing.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















.jpg)