Regulatory Support

Product Description
Vetter Pharma-Fertigung GmbH & Co. KG

-
DE
-
2015On CPHI since
-
3Certificates
-
5000+Employees
Company types
Primary activities
Categories
Specifications
Vetter Pharma-Fertigung GmbH & Co. KG

-
DE
-
2015On CPHI since
-
3Certificates
-
5000+Employees
Company types
Primary activities
More Products from Vetter Pharma-Fertigung GmbH & Co. KG (6)
-
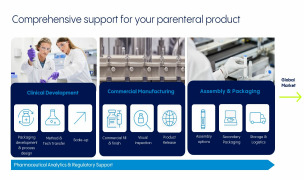
Product Drug Product Development
Our Vetter Development Services help biopharma customers lay a foundation of scalability, quality, and sustainable value for drug product profiles. We support drug developers in navigating key steps, decisions, and transitions in the product development cycle. This includes primary packaging evaluation, pr... -
Product Aseptic filling and visual inspection
For over 40 years, our fill and finish services have helped to keep the world supplied with injectable therapies, many of which are lifesaving. Our partnerships with customers start in the first steps of clinical manufacturing through commercial-scale filling and beyond, including management throughout the... -
Product Device Assembly and Packaging
The injectable drug market is rapidly evolving and includes an array of delivery devices from autoinjectors to pens and other intuitive formats. We provide partnership for drug owners driven by the top-quality, complex considerations and flexibility expected. These services include assembly process des... -
Product Analytical Services
From a drug product’s first raw materials to its release-ready batches, our experts rigorously evaluate and verify a product’s quality at key steps throughout the manufacturing process. Analytical services include material testing to verify product quality from the early stages of development, cGMP-com... -
Product Corporate Service Portfolio
We place heightened focus on your success as your strategic partner.
We produce aseptically prefilled syringes, cartridges, vials and dual-chamber systems as a globally operating CDMO partner. We are a family-owned, independent company rooted in 70+ years of history. We do not manufactu... -
Product V-OVS® next syringe closure system
V-OVS® next is a new evolution of the V-OVS® Luer Lock closure system: A product that has helped shape the market for 20+ years. Several innovative updates further enhance the system’s functionality, including an optimized opening mechanism and a streamlined design that fits an even wider range of syri...
Vetter Pharma-Fertigung GmbH & Co. KG resources (4)
-
News Vetter joins United Nations Global Compact Network
Vetter (Ravensbury, Germany), the aseptic filling and packaging CDMO has signed up to the UN Global Compact Network. -
Brochure Unlock the full potential of your injectable product
For over four decades, pharma and biotech companies around the world have relied on us to put their parenteral medications on a path to success. We’re proud to help our customers advance their valuable products with innovative services, cutting-edge technology, and flexible, robust, scalable processes that set our partnership apart. -
News CPHI Trend Report - CDMO Opportunities in the Chinese Market
Opportunities for contract development and manufacturing organisations (CDMOs) in China continue to grow but many questions remain over what the future of the sector will look like in the world's second largest pharmaceutical market. -
Webinar Evolution of Syringe Closure Technology: Bridging Experience With Innovation
This presentation looks at the crucial role of syringe closure systems in the field of prefilled syringes and injection devices, offering scientific insights into container closure development. With extensive experience of legacy closure systems, our company explores the potential of integrating historical knowledge with contemporary development methods to create a novel closure system. This presentation will showcase how efficient development strategies and state-of-the-art methods can be used to create a development program that is based on history, is driven by innovation and adheres to the latest combination product regulations.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance












-file155058.png)