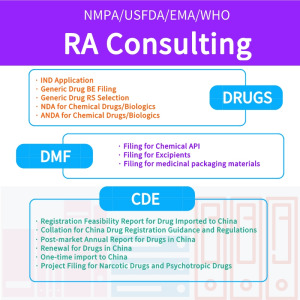
REGULATORY AFFAIRS

Product Description
Unica suporte científico e regulatório

-
BR
-
2019On CPHI since
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
Unica suporte científico e regulatório

-
BR
-
2019On CPHI since
-
1 - 24Employees
Company types
Primary activities
More Products from Unica suporte científico e regulatório (2)
-
Product GOOD MANUFACTURING PRACTICES
- Quality Audits
- Risk assessment and management
- Review of Documents
- Training
- Preparation of technical reports
-
Product CLINICAL RESEARCH
- Support for strategic evaluations and regulatory alignment on clinical trials
- Qualification of study centres and researches
- Preparation of research protocols
- Preparation of Clinical Drug Development Dossiers (CDDDs)
- Monitoring of clinical studies
- Review of scientific literature
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





















.png)