- Home
- Inpharmatis
- Regulatory Affairs
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Regulatory Affairs

Product Description
Inpharmatis offers comprehensive regulatory affairs services to Life Science Industry including Drug Development and Vigilance Services to pharmaceutical, medical device, food supplements, cosmetic and biotech companies. Our area of expertise lies in the European & CIS market, however though our network of credible partners we are able to manage also US, Latin America and selected RoW procedures. A multi-disciplinary team of qualified regulatory affairs consultants is at your disposal for all aspects of your marketing authorisation applications for active substances and human medicinal products. Inpharmatis shall provide full regulatory support over the whole life cycle of your product. - Advise on regulatory submission strategy - Development of regulatory strategy for the product’s life cycle- Submission preparation / review, filing and management - Regulatory Strategy Consulting - Filing and product life cycle management
Inpharmatis
-comp272482.gif)
-
LV
-
2018On CPHI since
Categories
Inpharmatis
-comp272482.gif)
-
LV
-
2018On CPHI since
More Products from Inpharmatis (2)
-
Product Pharmacovigilance services
Services of EU QPPV, Local QPPVPharmacovilgilance System Set up and Review servicesICSR managementOn-going monitoring and signal managementLiterature surveillance servicesRisk managementReporting – Product Safety Update Reports (PSURs) – Periodic Benefit-Risk Evaluation Reports (PBRERs) – Development Sa... -
Product eSolutions
Our eSolutions services include consulting services in all areas of Regulatory Operations, e.g. transition to electronic submission, compliance with EU eSubmission Roadmap, submission process optimisation, implementation of efficient Regulatory Information Management (RIM), compliance with XEVMPD and IDMP ...
Recommended Products
-
Product Development and engineering services by GEMÜ
GEMÜ engages with its customers at an early stage and supports them in all aspects of product development. From conceptual design, mould engineering, material consulting to sterilization and regulatory support, we can provide all competencies in-house. In addition, our developments are supported by sim...
-
Product Medical & Regulatory Services - Inhaled Products
Our formulation, device and development services are supported by our medical, regulatory, device vigilance and pharmacovigilance teams to safeguard your programme and ensure the smoothest path to product approval.
Our in-house team can support with:
Global regulatory development strategy...
-
Product Scientific Support
Selectchemie AG provides a wide range of services including scientific support. Our experienced professionals are at your service at locations in 18 countries to deliver tailor-made solutions according to your needs. Contact us for more information.
-
Product Pediatric Development
Pediatric formulation and product development
The development of acceptable, palatable pediatric formulations is a key feature within the industry today, driven by patient needs and regulatory requirements. Quotient Sciences has extensive knowledge and capabilities that enable us to provide you...
-
Product IT/EU REGULATORY AFFAIRS - Support and management of Technical documenta...
OP Pharma has been providing innovative services in the Regulatory Affairs field for more than 20 years to small to big pharma Companies in Italy as well as in the European Union.
Our expertise covers the lifecycle management of pharmaceutical products, whole or in part, ranging from digitization ...
-
Product Regulatory Support
Avéma Pharma Solutions can help guide your product successfully through NDA, ANDA, CBE 30 and 505(b)(2) filings with the FDA.
-
Product Regulatory Affairs
The field of RA encompasses all the work necessary to manage products' registrations and to receive and maintain marketing authorization.Choose PQE Group's comprehensive support and broad strategic knowledge to launch products without delays and keep them on different worldwide markets.
-
Product Commercialization Services
CARBOGEN AMCIS has a track record, extending over 25 years, helping our customers transition their molecules from process development through validation and ultimately into commercial status. A range of services backed up with fully integrated support functions has been developed to facilitate and smooth t...
-
Product R&D Laboratory of medical devices
Over 400 developed recipes,
including over 50 medical devices in various classes and indications,
in 20 pharmaceutical forms -
everything starts in the Gofarm's R&D Laboratory.
In Gofarm's R&D Laboratory our and our clients' ideas take real shapes...
-
Product Cortellis HTA Intelligence™
Increase successful submission outcomes in markets worldwide with comprehensive, expertly analyzed regulatory and market access information:
-Get treatments to patients faster Compare and monitor regulations quickly across markets to understand regional health technology assessment (H...
-
Product Formulation Development
Recipharm offer formulation development services for all dosage forms. We develop everything from simple formulations for early studies to more complex formulations suited for commercialisation.
-
Product DA Lawyers
Life Sciences at DA Lawyers cares of the legal business needs of your company in Spain. Highly specializes in pharma distribution, we are your legal partners in your NDAs, Customer Protection Agreements, Distribution Agreements, Supply and Manufacturing Agreements, Agency, compliance issues, customs and...
-
Product Development
Synerlab provides wide range of services which includes development. Contact us for more information.
-
Product Wasdell QP Services
Our dedicated QP Services team are able to provide compliance support, audit services, regulatory consultancy and QP certification for imported medicinal products.
-
Product Regulatory services
For pharmaceutical products, we offer support for regulatory services such as:
registration via national and European Union procedures;ANDA registration;
drafting of e-CTDs to create e-CTD sequences;
regulatory compliance;
minor and major variations (evaluation and/or management).For ... -
Product Regulatory & Market Access
With the growth in pharma manufacturing capability in Asia, each manufacturer nurtures an ambition to have their own footprint in the EU market.
PharmSol, with its own setups in Germany and Malta, has created a platform which provides a comprehensive, integrated and seamless market access and su...
-
Product Hi-End Resource On Demand Solutions
Project resource management includes forecasting the need to hire and/or outsource some tasks. When projects are high-value and require strategic decisions, in-house resources may be insufficient. Similarly, administrative action items are better left to contract workers so as to not overburden your staff....
-
Product Regulatory Affairs & Compliance Support
Thépenier Pharma & Cosmetics offers comprehensive regulatory affairs and compliance services to ensure that all products meet global pharmaceutical standards. From dossier preparation to liaising with health authorities, the team ensures smooth regulatory approval processes. With expertise in GMP, ISO ...
-
Product Biologic drug substance CDMO services
From pre-clinical development to commercial supply, Patheon by Thermo Fisher Scientific is an industry leader in the development and manufacture of mammalian cell culture drug substances. Patheon offers biotech and pharmaceutical companies the ability to pursue opportunities around the globe with a fully i...
-
Product CordenPharma Drug Product Support Services
SUPPORT SERVICES FOR OUR CONTRACTED CUSTOMERS • Analytical Development • Validation Support • Regulatory Support • Clinical Supply Services
-
Product Pharmaceutical drug substances (APIs)
Seratec specializes in fine organic chemistry and is dedicated to active pharmaceutical ingredient (API) development & production for the pharmaceutical industry.
Seratec operates in highly regulated markets and where high quality products are critical.
Seratec offers a complete range of cust...
-
Product Regulatory Affairs Services
Midas Pharma offers wide range of pharmaceutical services which includes regulatory affairs services. It offers regulatory support for human medicinal products, veterinary medicinal products and herbal medicinal products in Europe. This includes: • drug substance services: development of ASMF/E...
-
Product International Debt Collection
Debt collection activities all over the world: we support Top European Pharma Groups, with extra-performances -
Product Registration of Pharmaceutical products
Registration of Pharmaceutical products in Algeria, Middle east and north Africa (MENA)
-
Product Regulatory Services
Our regulatory services team has decades of experience in obtaining and maintaining market authorizations and registrations. We ensure compliance of medicinal products, medical devices and food supplements during their complete life cycle. Currently we obtain compliance in 55 countries worldwide. Conta...
-
Product Consulting: ICH Q10
Pharmaceutical companies must be capable of delivering products to the market with the utmost level of quality and safety. At Zamann Pharma Support, we have been aiding large and mid‐sized pharmaceutical companies in solving quality and compliance challenges while ensuring that practicality and qualit...
-
Product GMP Analytical development and validation services
Our state-of-the-art laboratories offer a wide range of analytical services to support your exact needs under strict quality procedures which meet GMP requirements and following ICH guidelines.
The analytical department provides support during the development of drug formulations combini...
-
Product Regulatory Affairs Support
At Pattern of USA Inc., we've achieved seamless local regulatory compliance in over 130 countries, with centralized management based in the U.S. and project-based teams comprised of local professionals. This ensures solutions are tailored to diverse international regulatory requirements.

-
Product Regulatory Support
Armed with decades of experience and a mature Quality System, we collaborate with you to ensure your medical device is market-ready and complies to all latest industry regulations.
Our capabilities and experience include:
- Fully integrated Quality System- Diversified & a...
-
Product FDA Compliance Monitor (Quality Assurance Tool)
Automate your supply chain management. The FDA Compliance Monitor allows you to easily identify vulnerabilities in your supply chain. You can monitor the status of your own or your suppliers' FDA registration numbers as well as monitor for recalls, inspection results, warning letters, imp...
-
Product Pharmacovigilance - PvEdge - AI Enabled Drug Safety Database Suite
AI-enabled, cloud-ready,360 pharmacovigilance solution for safety database management. An end-to-end globally compliant safety software for Drugs, Devices, Vaccines and Combinational products from case intake to submissions and reporting. • Case Intake • Case Processing • Case Submission • Risk Manage...
-
Product Medical Cannabis Consulting
Experts in management and assessment of the production of medicinal cannabis.
The regulation of the cultivation and production of cannabis derivatives favours the emergence of a new industry with a large global market .It is estimated that the market for medicinal cannabis could reach 5...
-
Product Anklam Extrakt Poduct Portfolio
• Premium Extracts – for your project Our extract portfolio includes both extracts in line with the ISO 22000 and/or Ph.Eur monograph for the pharmaceutical industry, and extracts for the food industry. From A for Althaea (marshmallow) to V for Vaccinium (cranberry) - we offer you a sophisticated and inn...
-
Product Regulatory Affairs
Our team of Regulatory affairs experts make our service offerings stronger.
Following are our Regulatory offerings:
• Regulatory Operations, Affairs
• Regulatory Intelligence, Information Management COTS
• Regulatory Strategy & Business Consulting
• Health Autho...
-
Product Pharma Regulatory Services
- Pre- and Post- Authorization Consultancy - Marketing Authorization Applications (NAP/DCP/MRP) - Life Cycle Management (renewals, variations etc.) - Submission of regulatory – related documents to Health Authorities - e-CTD submissions
-
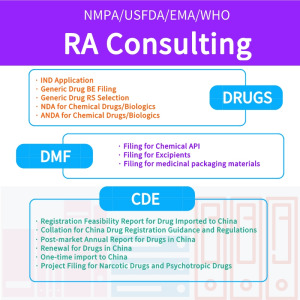
Product Regulatory affairs services
I. SERVICES 1. Submission of ANDA, NDA and IND applications with NMPA; (including the whole service from GAP analysis, compiling, submission, sample testing, CDE review procedure tracking and supplemental info preparation); 2. GAP analysis and application strategies setup before the submission to NMPA; 3. ...
-
Product IT/EU REGULATORY AFFAIRS - Support and management of RA practices
OP Pharma has been providing all-around services in the Regulatory Affairs field for more than 20 years to small to big pharma Companies in Italy as well as in the European Union.
Our expertise ranges from submission of registrations, variations, prices, texts, renewals (also via CESP), to CEP and...
-
Product REGULATORY AFFAIRS
- Regulatory support
- Political and social scenarios analysis within the Brazilian Health Sector- Market analysis to provide strategic information on the Brazilian Health System including public and private sectors
- Regulatory intelligence strategy
- Compliance
- Due diligence
-...
-
Product Cortellis Regulatory Intelligence™
Keep up to date with regulations, maintain biopharma regulatory compliance and make the right strategic decisions.
Navigate the regulatory landscape and efficiently drive strategic decisions
-Comprehensive repository of pharma regulatory intelligence Get full and time...
-
Product Clinical Supply
Recipharm have more than 20 years experience supplying clinical trial material to our clients. Our development facilities cater for a range of clinical trials from preclincal to smaller Phase III trials. We also offer commercial manufacturing for very large clinical batches.
-
Product Regulatory Affairs services
At Eignapharma we have a sound regulatory knowledge on the both national and international markets, providing support to our clients since 2010. Our experienced Regulatory Affairs team will listen to you and provide a tailored service, providing support to our clients in many different areas, suc...
-
Product Synthetic Chemistry
Recipharm offer a range of synthetic chemistry services through our global development facilities including advanced lead optimisation medicinal chemistry, process development and tech transfer to large scale GMP-manufacturing and other specialties.
-
Product Analytical Development
Recipharm offer analytical support for drug discovery, pharmaceutical development and manufacturing through our global development facilities.
-
Product Health, Safety and Environment Services
Our experienced HSE experts offer various health, safety and environment services: Safety data sheet (SDS) support (SDS classification, labelling) in most EU languages, including online access and QR links; Controlled substances, precursors, highly active substances and other restricted chemicals (in-clud...
-
Product Research & Development of medical devices
We provide a complete and comprehensive range of services from A to Z for substance-based medical devices: from product idea, through development and research, certification, registration and product delivery to the customer.
With us, you can easily launch a new substance-based ...
-
Product Cortellis HTA Intelligence™
Increase successful submission outcomes in markets worldwide with comprehensive, expertly analyzed regulatory and market access information 1.Get treatments to patients faster Compare and monitor regulations quickly across markets to understand regional health technology assessment (HTA) outcomes. 2.Main...
-
Product Regulatory Sciences
From early-stage development to post-approval, we partner with pharmaceutical, biotechnology, and medical device clients to overcome regulatory hurdles. Using science as the driver for success, we help our clients achieve positive regulatory outcomes with the Food and Drug Administration (FDA), Europea...
-
Product On-body Injector
Phillips-Medisize collaborated with Subcuject to bring a ground-breaking wearable bolus injector to the market. This device is designed to be a low-cost, patient-friendly, pre-filled wearable injector for single-use.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance































.png)