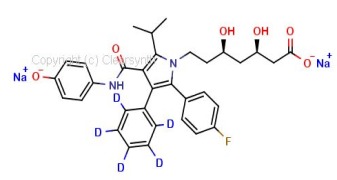
Rac-Hydroxy Ezetimibe D4
Product Description
CLEARSYNTH LABS LIMITED

-
IN
-
2015On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
CLEARSYNTH LABS LIMITED

-
IN
-
2015On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from CLEARSYNTH LABS LIMITED (99)
-
Product Brimonidine
a2-Adrenoceptor agonist. Antiglaucoma. -
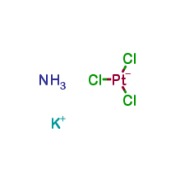
Product Cisplatin Impurity B
Potassium Trichloroammineplatinate is the degradation product of Cisplatin , an antitumor compound which exhibits mutagenic activity.;Impurity in commercial preparations of Cisplatin -
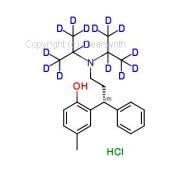
Product Raloxifene Hydrochloride
A nonsteroidal, selective estrogen receptor modulator (SERM). Antiosteoporotic. -
Product Tolterodine D14 Hydrochloride
Labeled Tolterodine, intended for use as an internal standard for the quantification of Tolterodine by GC- or LC-mass spectrometry. -
Product (S)-Lercanidipine D3 Hydrochloride
A dihydropyridine calcium channel blocker.;Labeled Lercanidipine, intended for use as an internal standard for the quantification of Lercanidipine by GC- or LC-mass spectrometry. -
Product Candesartan Cilexetil EP Impurity A
Impurity in commercial preparations of Candesartan. -
Product Racecadotril
Antidiarrheal; enkephalinase inhibitor, reducing the amount of water and electrolytes into the intestine. -
Product Selenous acid
It used as a source of SELENIUM, especially for patients that develop selenium deficiency following prolonged PARENTERAL NUTRITION. -
Product 4-Hydroxy Atorvastatin D5 Disodium Salt
A labeled metabolite of Atorvastatin,;Labeled Atorvastatin, intended for use as an internal standard for the quantification of Atorvastatin by GC- or LC-mass spectrometry. -
Product Clomiphene Citrate D5
Labelled Clomiphene. Synthetic estrogen agonist-antagonist. Gonad-stimulating principle.;Labeled Clomiphene, intended for use as an internal standard for the quantification of Clomiphene by GC- or LC-mass spectrometry. -
Product Domperidone EP Impurity A
Intermediate and metabolite of the gastrokinetic and antinauseant drug Domperidone. -
Product Sulfacetamide Sodium
An antibiotic that blocks synthesis of dihydrofolic acid by inhibiting the enzyme dihydropteroate synthase
CLEARSYNTH LABS LIMITED resources (2)
-
Brochure Corporate Profile
Your Globally ''Trusted'' Partner for Reference Standards and Research Chemicals. -
Brochure Deuterated Products
Clearsynth is pleased to be a global leader in deuterated chemistry. We manufacture from 'mg' to 'tonnes' in our state-of-the-art manufacturing facility.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance