R&D and Industrialization

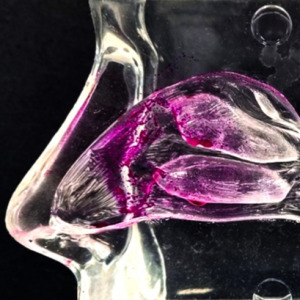
Product Description
C.O.C. Farmaceutici S.r.l.
-
IT
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
C.O.C. Farmaceutici S.r.l.
-
IT
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from C.O.C. Farmaceutici S.r.l. (3)
-
Product Contract Development and Manufacturing
We are a Pharmaceutical contract development and manufacturing company authorised for the preparation of medicinal products and medical devices in liquid, suspension, gel and cream form, lyophilisates and ointments. Our services include formula development, pilot studies in the laborato... -
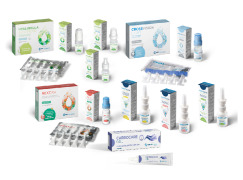
Product Products in Private Label
We offer customers a complete range of medical devices for nasal and eye care, ready for customisation with the graphics and brand of the distributor. They are CE-marked and can be sold in EU and non-EU countries, once they have been registered locally. -
Product Regulatory services
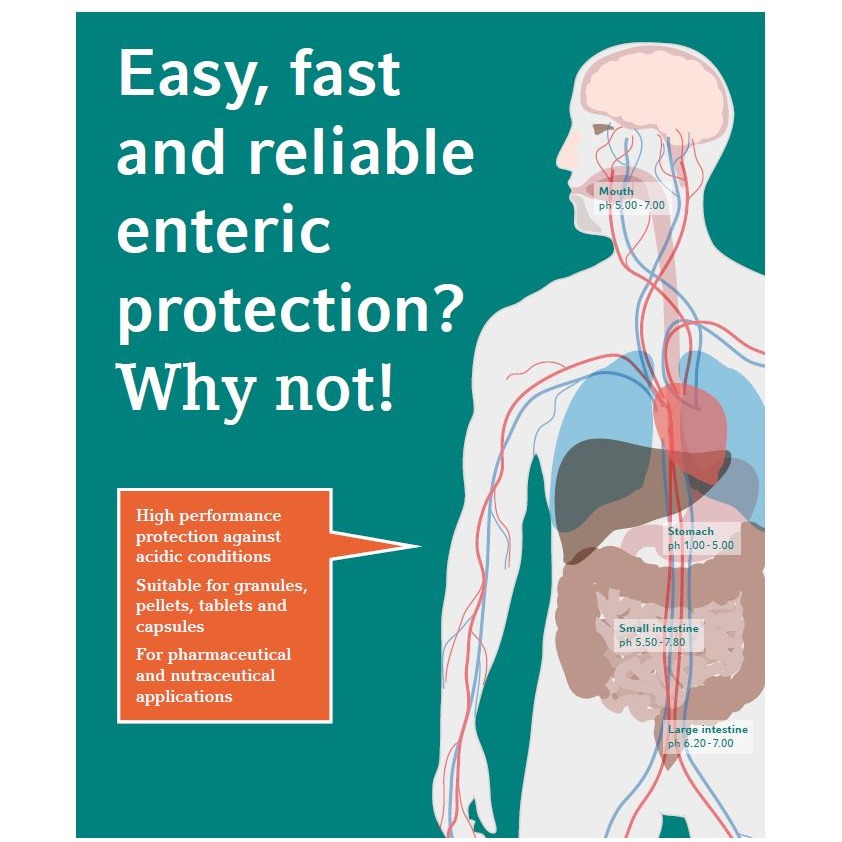
For pharmaceutical products, we offer support for regulatory services such as:
registration via national and European Union procedures;ANDA registration;
drafting of e-CTDs to create e-CTD sequences;
regulatory compliance;
minor and major variations (evaluation and/or management).For ...
C.O.C. Farmaceutici S.r.l. resources (2)
-
Brochure Contract manufacturing and contract filling services
Preparation, filling and packaging of pharmaceutical products, medical devices, cosmetics, products for veterinary and diagnostic in liquid, gel, cream, lyophilisates or ointments forms in a wide range of single-dose, multi-dose containers and tubes. -
Brochure Private Label products - product ready to be distributed
A complete range of medical devices for nasal and eye care, ready for customisation with the graphics and brand of the distributor. They are CE-marked and can be sold in EU and non-EU countries, once they have been registered locally.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










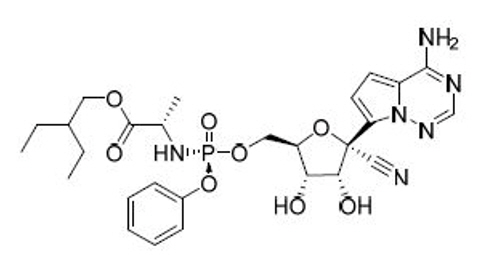































































%20(2).png)









-comp246544.jpg)