Product Development Services

Product Description
PharOS Ltd

-
GR
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
PharOS Ltd

-
GR
-
2015On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from PharOS Ltd (10)
-
Product CMO Services
CMO services could be offered for Oral Solid forms - especially for Oncology products - in PharOS manufacturing plant in Malta. -
Product Regulatory Affairs Services
PharOS Ltd. offers a wide range of services which includes regulatory affairs. Features: it enables to ensure that your dossier reaches marketing authorization status at the most time efficient and cost effective manner. Functional submissions outsourcing: applicable for pre-marketing submissions including... -
Product Business Development
PharOS Ltd. offers a wide range of services which includes business development. Features: it provides new opportunities and growth potential to clients. The experienced managers continuously seek, identify and assess individual products and potential new markets. The broad network of professionals, locate... -
Product Batch Release Services
PharOS has acquired a Certificate of GMP compliance from National Organization for Medicines for batch release of sterile and non-sterile products, as well as for imported medicinal products.
The PharOS team with its extensive experience offers to its clients batch release of their product... -
Product Chemistry Manufacturing and Controls (CMC) Services
PharOS Ltd. offers a wide range of services which includes chemistry, manufacturing and controls. Features: it uniquely qualified to assist in managing CMC needs. The skilled practitioners provide companies with the in-depth experience they need to ensure the robustness of their CMC capabilities. Dossier c... -
Product Pharmacovigilance Services
PharOS Ltd. offers a wide range of services which includes pharmacovigilance. Features: company's pharmacovigilance system (PVS)- qualified person for pharmacovigilance (qppv) from pre-approval to post-approval (24-hours service), provision of contact person for pharmacovigilance (in greece), to cover loca... -
Product Out - Licensing
Out-Licensing of Generic and other Value Added Pharmaceutical Products -
Product Contract Manufacturing Services
To learn more about our Contract Manufacturing Services in Malta, please contact: nfessa@pharosgr.gr -
Product Finish Dosage Dossiers for Out-Licensing
For our product portfolio please contact: businessdevelopment@pharosgr.gr -
Product EU eCTD Dossiers with Finished Product Supply
1. Out-licensing of our eCTD Dossiers covering various stability zones (including IVb), including supply of finished products (from manufacturing sites in Europe with various cGMP certifications from High Regulated Authorities worldwide)
2. Early and first to launch opportunities (generics)
3....
PharOS Ltd resources (2)
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















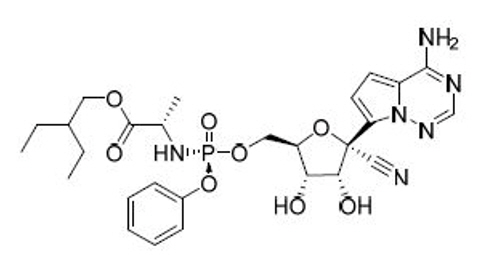












































![NexArni 50 [Valsartan with Sacubitril Tablets]](/46/product/129/06/57/picture.jpeg)