Process Development

Product Description
KBI Biopharma Inc.

-
US
-
2015On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
KBI Biopharma Inc.

-
US
-
2015On CPHI since
-
1000 - 4999Employees
Company types
Primary activities
More Products from KBI Biopharma Inc. (14)
-
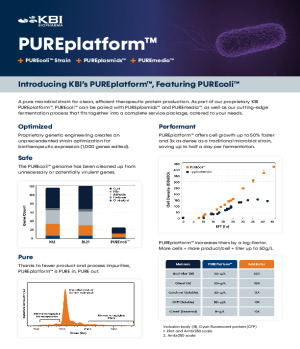
Product PUREplatform™
At KBI Biopharma, our PUREplatform is everything you need to make a protein. Containing plasmids, strains, media, and a fermentation process that all fit together into a service package catered to your needs.
• Features our PUREcoli™ cell line, our proprietary high-producing E. coli ce... -
Product SUREmAb™
Built on the SUREtechnology Platform, SUREmAb optimizes monoclonal antibody (mAb) development and manufacturing processes, fast-tracking the path to clinic and market. • Fast-tracked for efficiency and speed, taking you from DNA transfection to GMP drug substance in 11 months • Comprehensive mAb develop... -
Product SUREtechnology Platform™
With our advanced gene technology and proprietary cell line, we are able to overcome common bottlenecks that come with developing high-performance mammalian cell lines, especially when expressing novel molecule types.
Mammalian cells are the gold standard for biologics development.
... -
Product Biologic Drug Substance & Drug Product Formulation
To date, KBI has conducted over 130 successful protein, peptide, and vaccine formulation development programs. KBI's approach to formulation development is based on the strategic pairing of two complementary scientific disciplines: Establishing a comprehensive understanding of the thermal, physical, chemic... -
Product cGMP Protein and Antibody Purification
KBI Biopharma has extensive experience developing purification processes for a wide variety of biotherapeutics, including: -Monoclonal antibodies-Bispecific antibodies -Enzymes -Fc fusion proteins -PEGylated proteins -Complex glycoproteins KBI supports 40+ annual programs ranging from FIH (First-in-Huma... -
Product Cell Line Development
KBI offers a full suite of CLD across both mammalian and microbial expression systems. KBI can apply established mammalian recombinant protein expression systems to provide rapid and industry-leading cell line generation services using: CHO-M Selexis SUREtechnology, CHO-ZN, CHO-DG44, CHO-S, and CHO-K1 GS. ... -
Product Analytical Development
With top expertise in protein analytics, KBI has successfully completed over 3300 analytical projects for more than 300 customers and more than 130 distinct molecules.
Our experience includes antibodies (IgG1, IgG4, IgM, FAb, ADC, Fc fusion), enzymes, cytokines, growth factors, highly glycosylat... -
Product Formulation Development
KBI’s approach to formulation development is based on the strategic pairing of two complementary scientific disciplines:
• Establishing a comprehensive understanding of the thermal, physical, chemical, and conformational stability. • Employing statistical design-of-experiment (DOE) to evaluate main ef... -
Product Characterization
Scale-down process characterization studies performed using a qualified scale-down model are a critical part of licensure applications for biologics.
KBI has significant expertise in the design and execution of these studies to enable customers to define their process control strategy to take ... -
Product Clinical Manufacturing
KBI Biopharma offers a broad range of cGMP biologics manufacturing services to biopharmaceutical companies worldwide. Our capabilities include reliable manufacturing for preclinical and clinical supply.
KBI’s experienced team produces high-quality therapeutics and vaccines thro... -
Product cGMP Mammalian Production
KBI offers global cGMP Mammalian Production, and organizational highlights include: • First CDMO to implement 2,000L single-use cGMP operations • High throughput single-use technology (AKTA Ready XL, 2500L SUM, 12m2 TFF) • A broad range of cGMP biologics manufacturing services from pre-clinical thr... -
Product cGMP Microbial Production
The KBI Boulder facility has extensive experience in generating E. coli based expression systems for high-level expression of recombinant proteins, typically using T7 expression in the BL21(DE3) strain as a platform. Following demonstration of expression in shake flasks, fermentation development ...
KBI Biopharma Inc. resources (26)
-
News CPHI Barcelona 2023: Tackling the Pharma Talent Precipice – Part 2
This year at CPHI Barcelona (24–26 October, 2023) we sat down with C-suite executives and HR professionals to discuss the looming talent crisis in the pharmaceutical industry. With hybrid working persisting post-pandemic and a growing skills gap, how can the pharmaceutical supply chain adjust to a changing labour force? -
Whitepaper Navigating a New Standard in Microbial Protein Expression
An ideal therapeutic protein “factory” creates a pure target product with high titers from the start, which can minimize development risk, timelines, and cost. E. coli has long been implemented as a therapeutic protein factory due to its simplicity, tractability, and wealth of information characterizing the microbe.
While common E. coli expression strains alter as many as five genes to improve one aspect of recombinant protein expression, KBI has created a platform E. coli with about 1,000 genes altered and roughly 1 Mbp of DNA removed. The result is a PURE, efficient platform expression strain—PUREcoli™.
-
News Aaron Pilling Discusses the KBI PUREplatform Launch
KBI's PUREplatform™ is a game-changing microbial expression offering that redefines biopharmaceutical manufacturing.
Hear from Aaron Pilling, Ph.D., Senior Director of Process Development, as he discusses the KBI PUREplatform, a premium microbial CLD platform that delivers unmatched efficiencies. -
Video Titration Curve Modeling in Bioassay Potency Method Development
In the contract laboratory environment, the time constraint is ever-present and works against both the contract lab and the client or sponsor. This is especially true for the cell-based potency testing teams, where the assays have an infinite number of variables ranging from optimal growth conditions to the generation of the ideal titration curve to accurately measure any effect on the targeted mechanism of action. To overcome this ever-present hurdle, the Cell-Based Assay Team at KBI utilizes a titration curve modeling tool to effectively predict the cellular response to an agonist or antagonist. -
News Executive Team - About KBI & Selexis Operational Consolidation
Hear from our parent company JSR Life Sciences and our executive team about the operational consolidation of KBI and Selexis as one organization. Get their perspective on how this brings us one step further on the path of becoming the icon of next-generation CDMOs and how the benefits translates for our partners. -
Webinar BsAbs purification with affinity capture and mix-mode chromatography
Watch the webinar to see how KBI Biopharma and JSR Life Sciences have developed processes for the purification of bispecific antibodies using Amsphere™ A3 affinity chromatography followed by two polishing steps to eliminate undesirable homodimers and other impurities.
In this webinar, we discuss bispecifics purification, including:- Challenges of bispecifics purification
- Protein A affinity capture chromatography design
- Comparison of Protein A resin and alternative affinity capture resin for bispecifics
- Mixed-mode Chromatography design for bispecifics -
News KBI Strengthens Leadership With Executive Appointments
KBI announces four recent executive appointments to continue its momentum and vision of becoming a next-generation CDMO. Joining the company are Tony Fraij, COO; Shaguna Seth, Chief of Staff and Global PMO Leader; Katie Edgar, SVP of Corporate Strategy and Alliance Management; and Sarah Wakefield, SVP, Corporate Communications. -
Whitepaper Delivering on the Promise of Bispecifics: Antibody Development
A breakthrough approach to bispecific production via streamlined workflow; cell line development to GMP manufacturing. -
News Unveiling KBI PUREplatform™, a Game-Changing Microbial Offering
KBI announced the launch of KBI PUREplatform™, a premium microbial cell line development platform that features unmatched efficiencies for biopharmaceutical production. A culmination of decades of expertise, the platform addresses challenges, empowering customers to bring products to market cheaper and easier. -
Webinar Streamlined Development and Production of Bispecific Antibodies
There is a global need for high quality, effective off-the-shelf therapies that can be used immediately, while concurrently avoiding supply chain issues and mitigating process complexities. Bispecific antibodies (bsAbs) are constructed to help meet this need due to their ability to bind to two or more different epitopes, thereby allowing them to perform multiple discrete mechanisms of action.
This webinar will demonstrate a breakthrough platform approach that encompasses the efficient production of bispecific molecules in an integrated, streamlined way, from CLD (cell line development) to cGMP manufacturing. The leverageable integrated workflow from KBI Biopharma and Selexis generates high quality clinical bulk drug substances under accelerated timelines.
-
News KBI Appoints Marykay Marchigiani CFO and Sigma Mostafa, Ph.D., CSO
Durham, North Carolina (May 10, 2023) – KBI, a JSR Life Sciences company, today announced the appointment of Marykay Marchigiani as Chief Financial Officer and Sigma Mostafa, Ph.D., as Chief Scientific Officer. The additions will allow KBI to fulfill its growth strategy of becoming a next-generation CDMO. -
Webinar Development of Protein A Continuous Chromatography
In this Webinar, KBI Biopharma, in collaboration with JSR Life Sciences, introduces a hybrid approach to multicolumn continuous chromatography (MCC) that converts the Protein A (ProA) capture chromatography step to a continuous chromatography process, offering many of the advantages of the MCC process while avoiding the challenges associated with connecting process steps.
Rapid advances in intensifying upstream processes for biologics production to achieve higher titers have left downstream processing as a potential bottleneck in the manufacturing scheme. MCC is an emerging technology that can significantly reduce material costs and time in plant. This mode of chromatography can lead to shorter processing times and improve efficiency as well as productivity compared to a traditional batch process.
Here, we focus on the conversion of a batch ProA step using Amsphere A3 resin to a 4-column continuous process on a BioSMB system. -
News KBI Appoints David Stewart Site Head of Durham cGMP Facility
Stewart has more than two decades of biotech leadership experience and a proven record of delivering best-in-class supply for patients. He will oversee all operations at Patriot Park, Durham, North Carolina, driving deliverables for KBI’s global biopharmaceutical customers.
-
Brochure KBI Biopharma Extends Commercial Contract with Leading Pharmaceutical Co.
KBI Biopharma announced that it has extended and expanded its manufacturing contract with a leading global pharmaceutical company. -
News KBI Geneva Inaugural FIH Batch Released Five Months After Opening
In late 2022, KBI successfully released its inaugural FIH manufacturing batch in compliance with cGMP standards in its recently expanded Geneva mammalian cell manufacturing facility. This important milestone demonstrates the capabilities of KBI, allowing clients to accelerate clinical research timelines.
-
Brochure KBI Biopharma Recognized as a Leader in Six Categories
KBI Biopharma Recognized as a Leader in All Six Categories of the 2024 CDMO Leadership Awards -
News KBI Biopharma Announced as 2023 ISPE Facility Finalist
KBI Biopharma is thrilled to announce that its Patriot Park facility has been named a finalist in the 2023 ISPE Facility Of The Year Aawards (FOYA) in two categories: operations and pharma 4.0 -
Brochure KBI Biopharma and Argonaut Manufacturing Forge Strategic Alliance
KBI Biopharma, Inc. and Argonaut Manufacturing Services, Inc. Forge Strategic Alliance to Support Global Biopharmaceutical Companies -
News Subcontract with Mapp on Sudan ebolavirus Treatment
Following the recent Sudan ebolavirus outbreak in Uganda, KBI is providing emergency support through global production efforts.KBI announced it entered into a subcontract with Mapp for the continued development and manufacturing of MBP134, an experimental combination monoclonal antibody treatment for Sudan ebolavirus. -
Brochure KBI Biopharma Appoints Jean-Baptiste Agnus as Chief Business Officer
Jean-Baptist Joins the KBI Biopharma Team as Chief Business Officer -
News KBI Biopharma and Selexis SA Appoint J.D. Mowery as CEO
KBI Biopharma and Selexis, announced in April 2023 the appointment of J.D. Mowery as CEO. Widely respected as an innovative biotech leader with a nearly 25-year track record of successfully inspiring colleagues and building organizations, J.D. will lead KBI and Selexis into becoming a leader in the next CDMO generation. -
Brochure SUREmAb™ - mAb Development, The Way It's Meant to Be
Innovative approach to monoclonal antibody development. Powered by Selexis®. -
News Streamlined Development for Efficient Production of Bispecific Molecules: Connect to Frankfurt on-demand
In this Connect to Frankfurt session, Séverine Fagète, VP, Cell Line Development Services at Selexis (Geneva, Switzerland), and Brandon Brino (Durham, USA), Process Development Scientist and Group Leader at KBI Biopharma, present an overview of a streamlined process for the production of bispecific molecules. -
Brochure PUREplatform™ - Breakthrough Approach to Microbial Expression
Titers up to 50 g/L empowering cost-effective biomanufacturing -
News KBI Biopharma and Selexis to Operate as one Organization
KBI Biopharma and Selexis are consolidating as one organization to accelerate innovation and growth for global biopharma customers. The new structure will enable integrated, seamless solutions, from cell line development through process development, to clinical and commercial cGMP manufacturing for mammalian programs.
-
Video Rapid Timeline to Tox Starting with KBI Biopharma's SUREtechnology Platform™, Powered by Selexis
KBI Biopharma’s cell line development services, powered by Selexis®, have been at the forefront of innovation for the last two decades. We are introducing the latest version of proprietary SURE CHO-M technology, which uses a transposase-based semi-targeted integration method and provides high productivity and stable cell lines for therapeutic protein production, while exhibiting a doubling time of 15-17 hours. Using this transposase-based cell line, KBI Biopharma is now delivering tox material in as little as 6 months from transfection. The tox material will be generated with the top 6 clones. In addition, we are introducing an afucosylated host cell line for the generation of therapeutic proteins without any fucose residues. KBI Biopharma is also introducing a completely new targeted integration technology developed by our own next-generation sequencing software. This panel of new cell line offerings surpasses customer expectations and is set to establish new industry standards.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

.jpg)






.jpg)












.png)














-file153367.png)