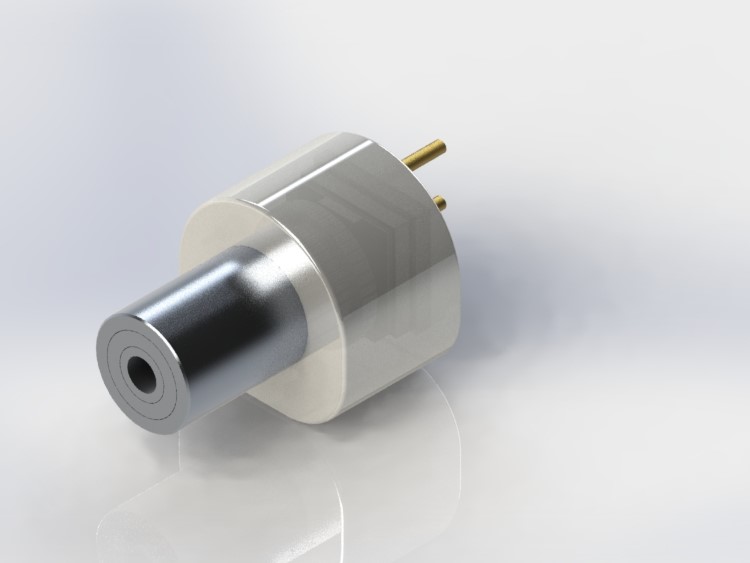
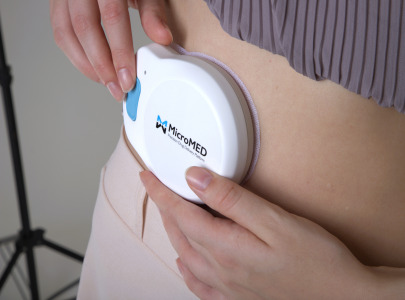
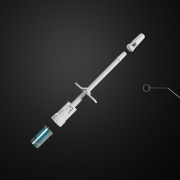
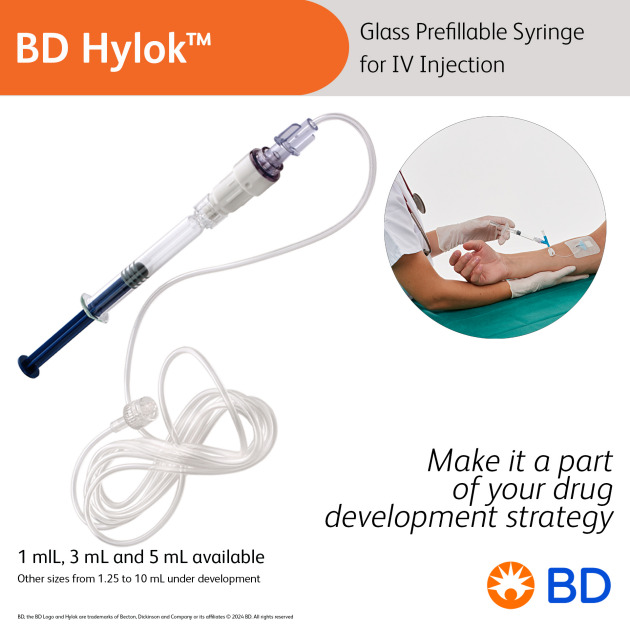
Primary Actuator For OBI/OBDS
Product Description
MicroMED Co., Ltd.

-
TW
-
2023On CPHI since
-
1 - 24Employees
Company types
Primary activities
Categories
Specifications
MicroMED Co., Ltd.

-
TW
-
2023On CPHI since
-
1 - 24Employees
Company types
Primary activities
More Products from MicroMED Co., Ltd. (2)
-
Product dBOBi™ on-body injector
The dual bag on-body injection platform:Compact, scalable, low-cost OBI for high-dose drug delivery
MicroMED has developed the dual bag on-body injector, a wearable on-body injection platform utilizing a Dual-Bag actuation approach. Powered by patented MEMS-enabled gas pump technology, it delivers subcu... -
Product Primary Actuator for Autoinjector
•Expanded range of volume selection: 3-10mL•Wide range of viscosity selection: 1-100cP
•Short delivery time: <30 seconds at 10mL
•Faster flow rate range: 0.06-0.9mL/s
•Utilization of rHuPH20 for high volumes (>3mL)
•Reusability for sustainability
•Usability enhancements through human-m...
Recommended Products
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance