Posaconazole
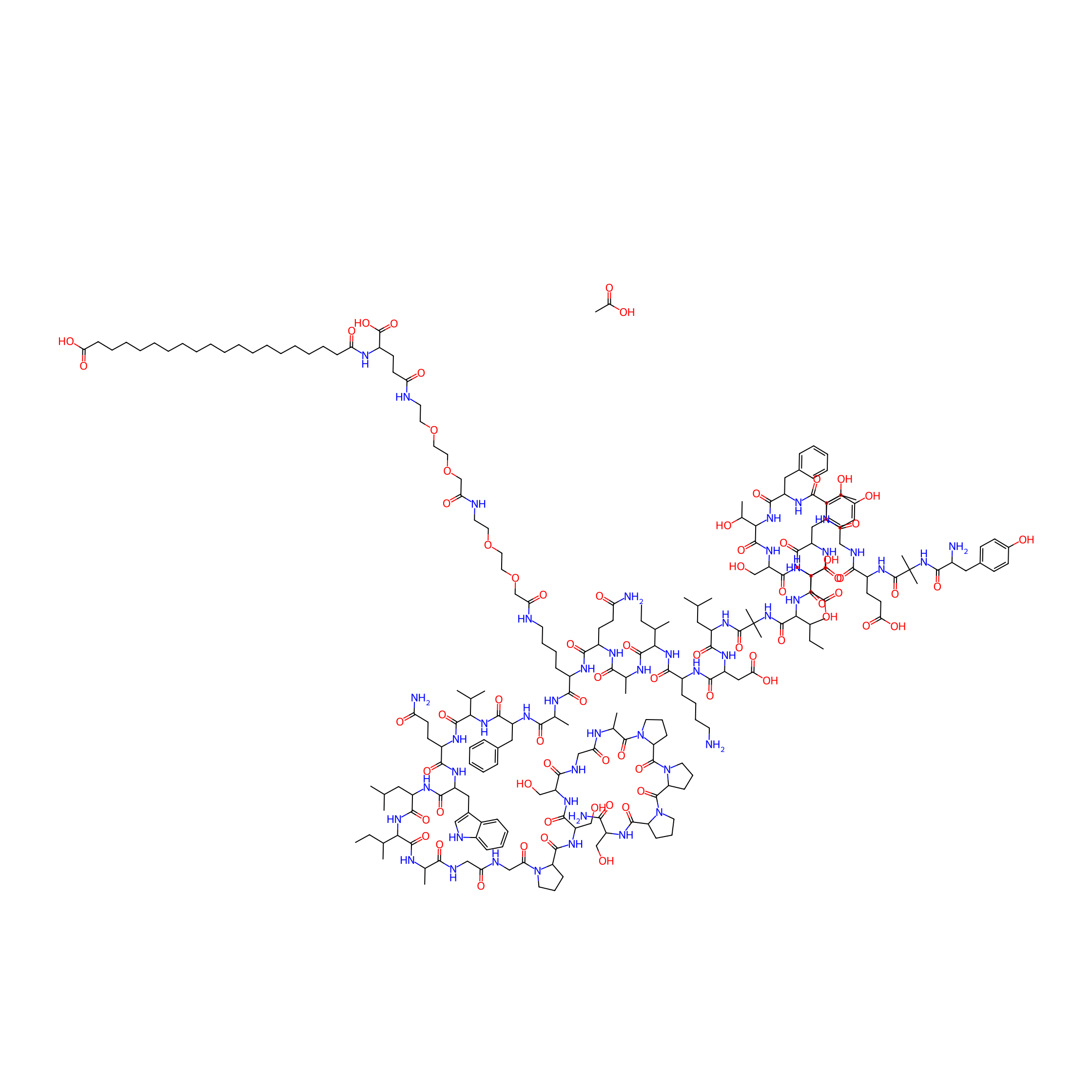
Product Description
Shenzhen Sijia Pharmaceutical Co., Ltd.

-
CN
-
2021On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
Specifications
Shenzhen Sijia Pharmaceutical Co., Ltd.

-
CN
-
2021On CPHI since
-
1Certificates
-
1 - 24Employees
Company types
Primary activities
More Products from Shenzhen Sijia Pharmaceutical Co., Ltd. (4)
-
Product Avibactam Sodium
Avibactam sodium is an organic sodium salt that is the monosodium salt of avibactam. Used in combination with ceftazidime pentahydrate for the treatment of complicated urinary tract infections including pyelonephritis. It has a role as an EC 3.5.2.6 (beta-lactamase) inhibitor, an antibacterial drug and an ... -
Product Everolimus
Everolimus is a macrocyclic lactone that is rapamycin in which the hydroxy group attached to the cyclohexyl moiety has been converted into the corresponding 2-hydroxyethyl ether. It is an immunosuppressant and antineoplastic agent. It has a role as an antineoplastic agent, an immunosuppressive agent, a mTO... -
Product Entecavir
Our company is committed to supplying API, advanced intermediates, excipients, chemicals, together with regulatory documentation support like DMF in CTD Format, from R&D to commercial production and cGMP audit service.
-
Product Ferric Carboxymaltose
Our company is committed to supplying API, advanced intermediates, excipients, chemicals, together with regulatory documentation support like DMF in CTD Format, from R&D to commercial production and cGMP audit service.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




