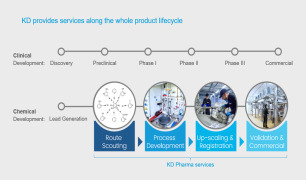
Pilot plant and manufacturing service

Product Description
PharmaBlock (USA) Inc.

-
US
-
2017On CPHI since
Company types
Specifications
PharmaBlock (USA) Inc.

-
US
-
2017On CPHI since
Company types
More Products from PharmaBlock (USA) Inc. (4)
-
Product Building blocks
Pharmablock sciences offers building blocks. Features: it is specially designed based on structural information of marketed drugs, clinical and preclinical candidates. It product series includes cyclopropane series; cyclobutane, azetidine & oxetane series; pyrrolidine, cyclopentane, tetrahydrofuran & pyraz... -
Product Custom synthesis service
Pharmablock sciences offers custom synthesis service. Features: it includes high-quality and cost-effective custom synthesis service with quantities ranging from grams to tons. Contact us for more information. -
Product GMP manufacturing
505 m3 GMP manufacturing capacity in ChinaGMP capabilities available in Pennsylvania, USA -
Product Process development service
Pharmablock sciences offers process development service. Features: it includes creative synthetic routes scouting, chemistry process development and optimization, impurity identification and profiling, technical package transfer. Contact us for more information.
PharmaBlock (USA) Inc. resources (1)
-
Video CPHI Milan Interview with PharmaBlock on ACS Green Chemistry win
Dr Jing Li, President; Senior VP of Process Chemistry of PharmaBlock (USA) Inc., speaks in an interview from CPHI Milan 2024 on their recent win for the ACS Green Chemistry Award. PharmaBlock is invested in innovation for sustainability, PharmaBlock has a strong vision to drive the sustainable development of the company and the industry via innovation in green chemistry and low-carbon manufacturing. The interview covers the following questions: What motivated PharmaBlock to establish a vision centered on sustainable development through green chemistry and low-carbon manufacturing innovations? How does this align with the company's long-term goals and industry trends? Congratulations to PharmaBlock on winning the ACS Green Chemistry Award for two consecutive years, as the only CDMO company winner globally. PharmaBlock is known for its application of low-carbon technologies like continuous flow and biocatalysis. How do these technologies contribute to sustainability in pharmaceutical manufacturing, and what sets PharmaBlock apart in this area?
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















%20png-comp246344.png)











![Fomepizole [7554-65-6]](/46/product/124/54/03/p1245403th_S.jpg)









-comp306407.jpg)













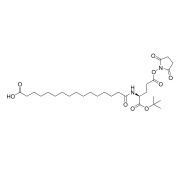








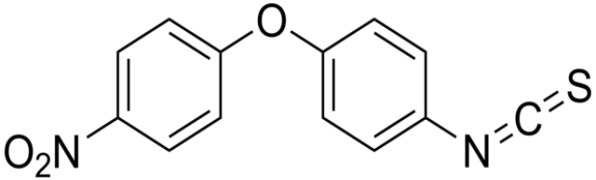

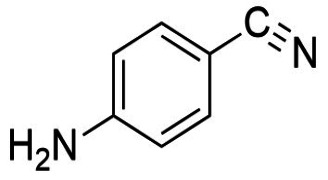




![2-Fluoro-5-[(3-oxo-1(3H)-isobenzofuranylidene)methyl]-benzonitrile](/46/product/68/69/16/p686916th_S.jpg)
















%20(1).jpg)

-comp270857.png)