PiccoJectâ„¢ Autoinjector

Product Description
Haselmeier GmbH

-
DE
-
2021On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Haselmeier GmbH

-
DE
-
2021On CPHI since
-
3Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from Haselmeier GmbH (11)
-
Product In-Pen Reconstitution
With one approved product and several more in development, Haselmeier has experience with multiple solutions that allow easy and convenient administration of freeze-dried biologics.
In-Pen Reconstitution with a Reusable Pen In this solution, each drug cartrdige is supplied with a disposable cartridge ... -
Product Re-Varioâ„¢ & Re-Varioâ„¢ A Injection Pens
The Re-Vario is a re-usable pen platform with a anodized aluminium body to provide years of reliability. The Re-Varioâ„¢ A is a high quality, plastic re-usable pen.
The product platform (Re-Varioâ„¢ / Re-Varioâ„¢ A) is the basis for a re-usable, variable dose injection device designed fo... -
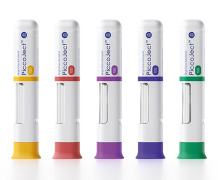
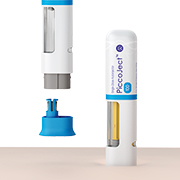
Product PiccoJect Autoinjector
PiccoJect is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.
• Extremely low part count: Only 8 parts in total – reduces manufacturing and scale up challenges • Ergonomic shape & small size: Thanks to its compact flat f... -
Product D-Flex Injection Pen
D-Flex, the pen platform for subcutaneous self-injection. Bridging the gap between fixed and variable-dose pens, this innovative product also paves the way for connected commercial devices.
• Just one pen for many needs: Disposable pen for fixed or multiple fixed dosages • The versatile solution: Allo... -
Product Re-Vario™ & Re-Vario™ A Injection Pens
The Re-Vario is a re-usable pen platform with a anodized aluminium body to provide years of reliability. The Re-Vario™ A is a high quality, plastic re-usable pen.
The product platform (Re-Vario™ / Re-Vario™ A) is the basis for a re-usable, variable dose injection device designed for use... -
Product Certified drug manufacturing
As a full-service provider, Haselmeier can support you with a lot more than just R&D and product development. We also provide certified pharmaceutical manufacturing services, and so are able to offer pharmaceutical and biotechnological companies a comprehensive assembly, labeling and packaging service. A... -
Product D-Flex(TM) Connect
D-Flex(TM) Connect allows smart data management for therapy efficiency, offers great opportunities to develop customized solutions for clinical studies and supports a fast pathway to connected commercial devices. THE D-FLEX(TM) CONNECT IS SMART-HEALTH READY Haselmeier's D-Flex(TM) Connect solution c... -
Product D-Flex(TM) LOGBOOK
The D-Flex(TM) LOGBOOK covers your needs from clinical testing to commercial launch. It allows utilization of a commercially viable injection pen already during dose ranging trials. Its Dose Selector Technology enables quick adoption to different dose settings and once reaching commercial launch there is n... -
Product For speed and flexibility in clinical trials
Haselmeier's D-Flex(TM) platform is the ideal choice for use in clinical trials and beyond, supporting you from drug development, through clinical trial, to commercial market launch. A complete solution offering for pharmaceutical companies and biotech start-ups To be deemed suitable for use in clinical... -
Product Our Product Platforms
Our innovative product platforms and related service portfolios assist you every step of the way - leading to an advanced combination of drug and device that is as time-saving and economical for your company as it is safe and convenient in therapy. We support you at every stage - from technology to therapy... -
Product Our Service - Product Development
We offer two principal product development pathways: customized pens and platform pens. Platform pens - a fast, flexible development approach We work together with you to develop unique customized pen solutions that reflect the specific requirements of both you and your patients. We can develop...
Haselmeier GmbH resources (20)
-
Webinar Case Study: Importance of Simulation and Modeling in Autoinjector Development
In this webinar, originally broadcast as part of Pharmapack Europe 2022, Chris Muenzer, Vice President of Innovation & Development, Haselmeier, a medmix Brand discusses how development of an integrated drug delivery device is a complex exercise that requires expertise in medical device engineering and pharmaceutical science. Health authorities expect legal manufactures to have a strategy to control device performance through the entire supply. Modeling and simulation is an important tool to ensure robust and repeatable performance while maintaining time to market. This case study looks at how Haselmeier is applying these techniques during the creation of a new autoinjector platform. In addition, Chris will look at future opportunities for further improvements though expanded use of digital twins and other technologies. -
Video PiccoJect Video
PiccoJect™ is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.Extremely low part counrtLarge wrap-around drug window with customizable sizeOptimized spring forcesFuture proofCommitted to sustainabilityErgonomic shape & small sizeColored status indicatorAudible clicks at the start and end of injectionAny standard 1 ml or 2.25 ml pre-filled syringeA full-service platform
The products shown in this video are under development and some of them may not yet have been approved for sale under applicable medical device regulations. The concept provides general information about these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice. -
Brochure PiccoJect Product Brochure
PiccoJect™ is a highly compact, customizable and fully featured two-step autoinjector designed for subcutaneous delivery of drug products.Extremely low part counrtLarge wrap-around drug window with customizable sizeOptimized spring forcesFuture proofCommitted to sustainabilityErgonomic shape & small sizeColored status indicatorAudible clicks at the start and end of injectionAny standard 1 ml or 2.25 ml pre-filled syringeA full-service platform
The products shown in this video are under development and some of them may not yet have been approved for sale under applicable medical device regulations. The concept provides general information about these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice. -
Whitepaper Excellence through Simplicity – PiccoJect™ Autoinjector
Effective self-management of chronic diseases requires healthcare solutions that are easy and convenient for patients. This is one of the key drivers for our patient-focused development of innovative drug delivery solutions.
The simplicity of PiccoJect™, combined with our sustainability philosophy, is reflected in the entire supply chain to reduce waste and minimize environmental impacts. In addition to investing in green electricity and the use of sustainable feedstocks, we focus on the development of regional supply chains for the US and European markets. 'Excellence through simplicity' sums up our patient-centered and sustainable philosophy.
The products shown in this video are under development and some of them may not yet have been approved for sale under applicable medical device regulations. The concept provides general information about these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice. -
Whitepaper Whitepaper - INNOVATIVE INJECTION PEN - THE D-FLEX™
The D-Flex™ closes a gap in the market: The D-Flex™ injection pen can do more than previous pens. D-Flex™ can be configured for several fixed dose values and is thus a variable fixed dose pen. It makes it possible to integrate a certain dosage value or several different fixed dosage volumes in one injection device. -
Video Manufacturing Site Dnesice, Czech Republic
Haselmeier Manufacturing Site Dnesice, Czech Republic -
Video D-Flex™ Ecosystem - From data to value
The D-Flex™ Ecosystem comprises hardware, software and services enabling data capture in clinical trials. In particular, the system consists of the D-Flex™ injection pen, a digital cap, a mobile app and the Haselmeier™ dataplatform. The digital cap captures data at the point of care and securely transfers it to the Haselmeier™ data platform.The platform allows controlled and secure access to the different stakeholders. -
Video Manufacturing Site Bengaluru, India
Haselmeier Manufacturing Site Bengaluru, India -
Brochure D-Flex™️ Connect - The connected solution with a digital cap
Operating with patented, Bluetooth (BLE)-enabled, dose-sensing technology, the digital cap measures the dispensed dose, time and temperature, enabling seamless data collection at the point of care. D-Flex™️ Connect is a proven technology that can be adapted to a number of drugs and indications. -
Brochure D-Flex™ Ecosystem - From data to value
Here, Fred Metzmann, PhD, Director Business Development, and Frank Leipold, Business Developer Digital Solutions, at Haselmeier, discuss the benefits of connected medical devices in clinical trials and describe the advantages of Haselmeier's D-Flex® Ecosystem for improving adherence and reducing the dropout rate in clinical trials. -
Brochure DEVELOPMENTS IN SELF-INJECTION DEVICES FOR COMBINATION PRODUCTS
In this article, Fred Metzmann, PhD, Director Business Development, reports on the latest state-of-the-art developments in innovative injection devices for combination products in subcutaneous self-application. He outlines Haselmeier's product platform strategy for single-use (D-Flex) and re-usable (i-pen²) injection-pen systems, and related services. He also looks at the company’s connected digital solutions, which support point-of-care data collection and transfer by patients, as well as data management by stakeholders in the care environment. -
Whitepaper Whitepaper - The D-FLex™ Injection Pen for GLP-1 Medication
Type 2 diabetes is a global health problem – according to expert estimates, the number of patients worldwide will increase by 48 percent by 2045. constantly elevated blood sugar levels mean stress for the blood vessels,which can lead to feared complications,including life-threatening sequelae such as a heart attack, organ failure or stroke. A consistent treatment of type 2 diabetes as early as possible can significantly improve the quality of life for the patient and the course of the disease.All patients have in common that they have had to treat it on their own their whole lives. A patient-centered approach must be consistently developed so that the GLP-1 analogs treatment is efficient.The innovative D-Flex™ injection pen can make a significant contribution to this. -
Whitepaper Whitepaper - Injection pens for osteoporosis therapy may contribute to greater compliance
The International Osteoporosis Foundation cites figures of around 200 million osteoporosis patients worldwide.Numerous patients suffer not just one bone fracture, but several at once. Treatment does not appear to be having the best possible effect. As an experienced partner in the design and production of injection systems, Haselmeier is also well prepared in terms of injection pens for osteoporosis therapy. -
Webinar Sustainable Drug Delivery: The Device Supplier's Perspective
This session will explore: The role of the device supplier in overall product sustainability Benefits of lifecycle assessment (LCA) in device development Trade-offs between usability, cost of goods, and carbon footprint Challenging Pharma to close the circle and enable better solutions -
Webinar Advanced Delivery Device Technology to Simplify the Reconstitution of Lyophilized Drugs
Lyophilization (freeze drying) is a proven process for increasing the shelf life of vaccines, biologics and other injectables. But delivering these products requires several use steps that make them challenging to administer, particularly by patients and caregivers in non-clinical settings. To address these needs, Haselmeier has been developing several solutions for an easier and more convenient reconstitution and administration. This presentation will cover different types of primary drug containers such as vials and dual chamber cartridges for reconstitution along with the available device solutions to simplify delivery. The challenges and advantages of each solution, including vial to cartridge, out-of-pen reconstitution, and in-pen reconstitution will be discussed. -
Video D-Flex Logbook
Simple patient monitoring for self-injection-based therapies
The D-Flex™ Logbook collects injection data at the point of care without the need for a patient mobile app.
D-Flex™ Logbook – From data to value
The D-Flex™ Logbook consists of the disposable D-Flex™ injection pen and a connected cap, which replaces the standard cap of the pen. Patients remove the connected cap from the injection pen, dial a dose, self-inject and put the cap back on the pen; exactly how they would use a pen and a standard protective cap. Once the patient returns the cap onto the pen, the connected cap automatically identifies the administered dose, the current temperature and time and stores this injection event in its internal memory. The cap can store up to 1,000 injection events.
The products shown in this video are under development and some of them may not yet have been approved for sale under applicable medical device regulations. The content provides general information about these products, their field of application and intended use, and their function, performance and specification are subject to customer specific development and may deviate from those shown herein. All information contained herein is directed at medical device manufacturers and pharmaceutical companies. This information shall not constitute a promotion for use in a way which conflicts with any applicable medical device regulations, nor is it directed at patients or intended to replace professional medical advice. -
Video D-Flex Logbook
Test -
Video D-Flex Logbook
D-Flex Logbook -
Video Application and Benefits of Connected Medical Devices in Healthcare
This webinar will address: Introduction of HM digital roadmap Presentation of a new smart drug delivery system, which does not require a patient phone/app – the D-Flex Logbook Value of smart drug delivery solutions in clinical testing This webinar originally aired as part of Pharmapack Europe 2021 -
Video Future Trends in Injection Device Development
This webinar will discuss: Past trends in injection devices Challenges in the industry Impact of platforms, digitalization, and sustainability Ways to change how we develop devices This webinar originally aired as part of Pharmapack Europe 2021
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-file120875.jpg)






-file145616.png)




-file122526.png)
-file122519.png)