Pharmaceutical Impurity Standard Synthesis
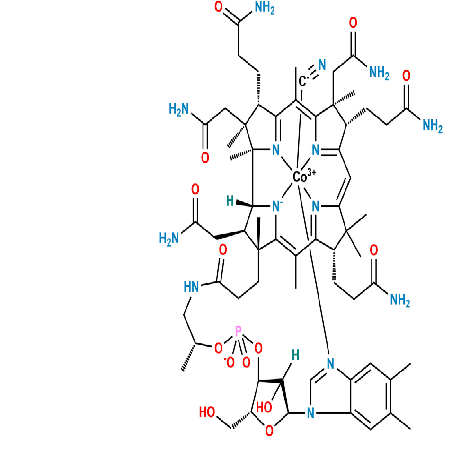
Product Description
K. M. Pharma Solution
-
IN
-
2023On CPHI since
K. M. Pharma Solution
-
IN
-
2023On CPHI since
More Products from K. M. Pharma Solution (4)
-
Product Active Pharmaceutical Ingredients (API's) and Intermediates
Febuxostat, Apixaban, Gliclazide, Clotrimazole, Deferasirox, Oxcarbazepine, Carbamazepine, Lidocaine, Prilocaine, Trazodone, Levocloperastine Fendizoate, Blonanserine, Rivaroxaban, Edoxaban, Flupirtine Maleate, Chlorthalidone, Duloxetine Hydrochloride, Fimasartan, Brivaracetam, Bempedoic Acid, -
Product Certified Pharmaceutical Reference Standards
We have more than 10000 Certified Pharmaceutical Reference Standards available Ready to Ship with all support data like, COA, HPLC/GC, Mass, 1H and 13C NMR, IR and TGA, Product like, Atorvastatin, Ezetimibe, Febuxostat, Apixaban, Doxazosin, Amlodipine, Mebendazole, Dapagliflozin, Empagliflozin, Bilastin, B... -
Product Nitrosamine Impurities
We have more than 1200 Nitrosamine impurities of Drug Substance and their intermediates Ready to ship -
Product Personal Care
Piroctone Olamine, Mexoryl XL, Octocrylene, Mexoryl SX, Tinosorb, Avobenzone, EHT (Ethylhexyl triazone)
K. M. Pharma Solution resources (3)
-
Brochure Brochure
We involved in synthesis of nitrosamines impurities, pharmacopeial and non-pharmacopeial impurity standards, reference Standards, Drug Metabolite -
Brochure Nitroso Impurity Product List
We are Expert in synthesis of nitrosamines impurities. -
Brochure Pharmacopeial and non-pharmacopeial impurity standards
K M Pharma Solution Pvt. Ltd. (CIN: U72100GJ2023PTC141278) is a chemistry based innovation driven company involved in synthesis of pharmacopeial and non-pharmacopeial impurity standards, reference standards, drug metabolites, glucuronides, process degradants, excipient related impurities, nitrosamines, unknown impurity isolation and characterization and stable isotope products.
Also is an integrated contract research, development and manufacturing organisation providing scientific services -- from early discovery to commercial supply. Our innovative capabilities for novel molecular entities (NMEs) cater to pharma, biotech, nutrition, animal health, consumer goods and speciality chemical companies.
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






