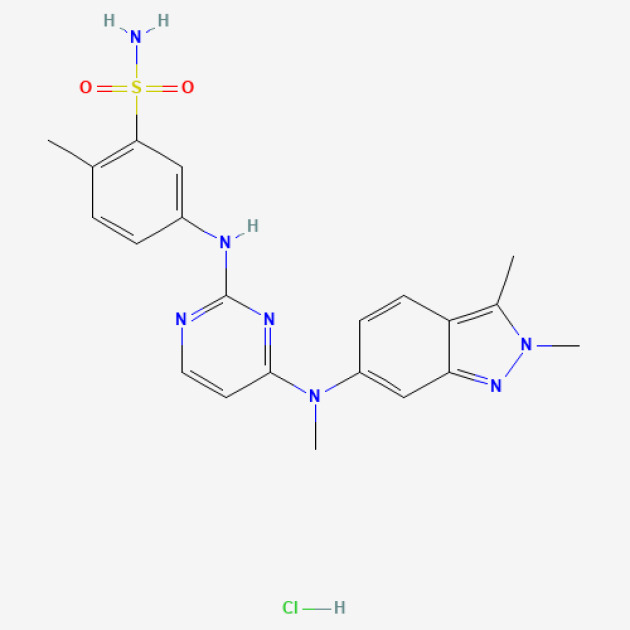
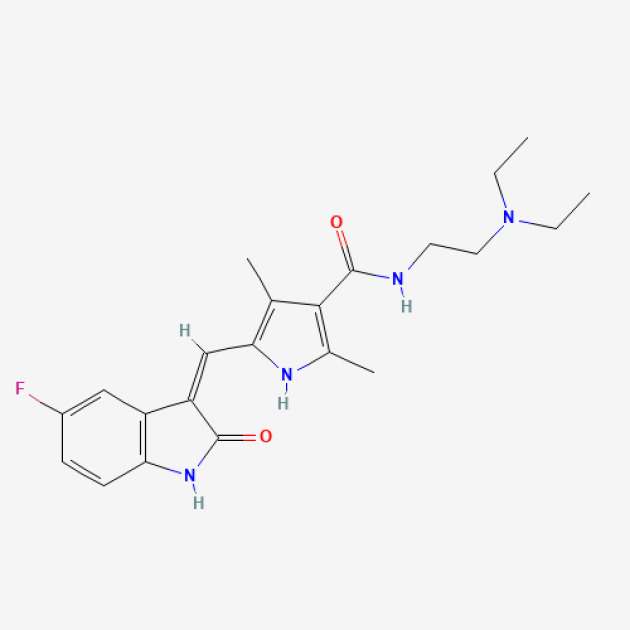
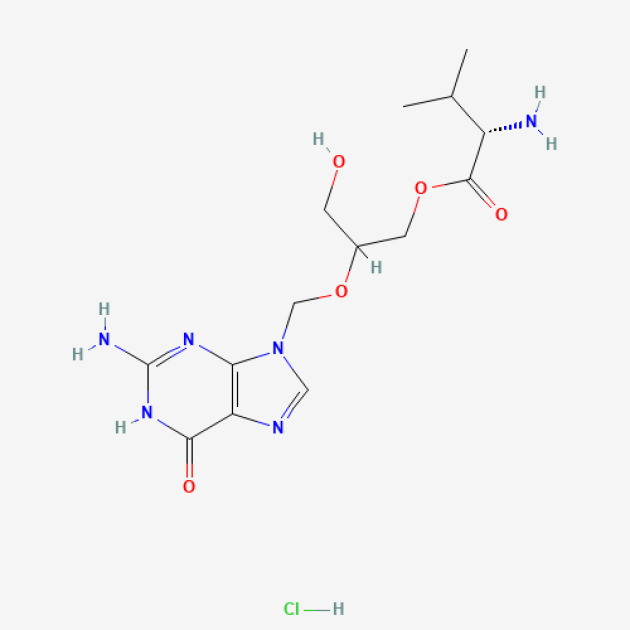
Pazopanib-PAZOTRI®
Product Description
EXAMON Handelsges.m.b.H.

-
AT
-
2024On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
Categories
Specifications
EXAMON Handelsges.m.b.H.

-
AT
-
2024On CPHI since
-
3Certificates
-
100 - 249Employees
Company types
Primary activities
More Products from EXAMON Handelsges.m.b.H. (31)
-
Product Pazopanib hydrochloride-API
• High-purity active pharmaceutical ingredient engineered for pazopanib-based formulations. • Offered as a fine crystalline powder with robust purity and consistent physicochemical properties. • Produced under rigorous cGMP standards to ensure batch-to-batch reproducibility and quality assurance. • Sub... -
Product Sunitinib malate-API
• High-quality active pharmaceutical ingredient developed for sunitinib-based formulations. • Provided as a fine crystalline powder with exceptional purity and consistent physicochemical attributes. • Manufactured under strict cGMP conditions to ensure uniform quality and batch-to-batch reproducibility.... -
Product Valganciclovir hydrochloride-API
• Premium-grade active pharmaceutical ingredient formulated for valganciclovir-based products • Supplied as a finely milled crystalline powder with exceptional purity and consistent quality • Produced under strict cGMP conditions to ensure reproducibility and high manufacturing standards • Extensively ... -
Product Erlotinib-KIMOTAR®
• Erlotinib blocks the epidermal growth factor receptor’s tyrosine kinase to suppress tumor cell growth. • Validated efficacy in non-small cell lung cancer (first-line). • Offered in tablet form to facilitate patient-friendly, outpatient treatment. • Underpinned by a wide range of peer-reviewed studies... -
Product Imatinib
• Targeted mechanism that selectively inhibits the BCR-ABL tyrosine kinase to disrupt signaling pathways driving abnormal cell growth in chronic myelogenous leukemia and gastrointestinal stromal tumors (first-line). • Clinically established with robust trials confirming its ability to control disease pro... -
Product Gefitinib-GEFISA®
• Used for non-small cell lung cancer (NSCLC) as first-line therapy. Targets EGFR mutations to inhibit tumor growth. • An EGFR tyrosine kinase inhibition which interrupts receptor signaling to limit tumor cell proliferation. • Robust clinical studies confirm benefits in non-small cell lung and pan... -
Product Sunitinib-KITENT®
• Multi-targeted tyrosine kinase inhibitor that blocks VEGF and PDGF receptors to hinder tumor angiogenesis and cell proliferation • Proven efficacy in advanced renal cell carcinoma (second-line), gastrointestinal stromal tumors (first-line), and pancreatic neuroendocrine tumors (second-line) • Administ... -
Product Fampridine(Dalfampridine)
• Fampridine acts by blocking voltage-dependent potassium channels, thereby enhancing conduction in demyelinated nerve fibers. • Robust clinical trials have confirmed its ability to improve walking speed and overall mobility in multiple sclerosis patients. • Delivered in tablet form, ensuring convenient... -
Product Azacitidine-Kimia
• Incorporates into nucleic acids to induce DNA hypomethylation. • Clinically validated for myelodysplastic syndromes and AML. • Flexible administration via subcutaneous injection or IV infusion. • Established safety profile confirmed in rigorous clinical trials. • Demonstrated improvements in surviva... -
Product Bortezomib-Kimia
• Inhibits the 26S proteasome, triggering targeted tumor cell apoptosis. • Clinically indicated for multiple myeloma and mantle cell lymphoma. • Offers flexible IV and subcutaneous administration options. • Demonstrated improvements in survival and quality of life. • Established, well-characterized sa... -
Product Calcium folinate (levoleucovorin)-Kimia
• Supports DNA synthesis and repair while mitigating methotrexate toxicity. • Proven efficacy in enhancing fluorouracil therapy for colorectal cancer and serving as methotrexate rescue. • Enhances chemotherapeutic regimens to optimize patient outcomes. • Demonstrated tolerability and manageable adverse... -
Product Carboplatin-Kimia
• Disrupts tumor DNA through crosslink formation to impede cell replication. • Widely validated in the treatment of various solid malignancies, including ovarian and lung cancers. • Administered via infusion to integrate seamlessly into contemporary oncology protocols. • Exhibits manageable adverse eff...
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance