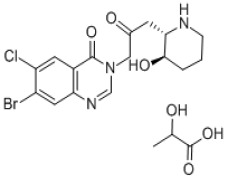
Palonosetron Hydrochloride
Product Description
Hangzhou Jiuyuan Gene Engineering Co.,Lt

-
CN
-
2015On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
Categories
Specifications
Hangzhou Jiuyuan Gene Engineering Co.,Lt

-
CN
-
2015On CPHI since
-
5Certificates
-
1000 - 4999Employees
Company types
More Products from Hangzhou Jiuyuan Gene Engineering Co.,Lt (16)
-
Product Semaglutide
Diabetes and Obesity -
Product Filgrastim
Filgrastim, Neupogen, G-CSF, Indicated to neutropenia, Formulated bulk liquid, l Injection (ampoule/PFS/vial) 75µg/unit, 150µg/unit, 300µg/unit, 450µg/unit
-
Product Fosaprepitant dimeglumine
cancerbrhrbrEmend, CAS number: 265121-04-8 -
Product Dulaglutide
diabetes (recombinant route), API -
Product Fulvestrant
an anti-cancer drug, Bulk and injection (PFS): 250mg in 5ml
-
Product Palonosetron Hydrochloride
anti-emesis after chemotherapy or radiotherapy, Active pharmaceutical ingredient (API) , Injection (ampoule): 0.25mg/5ml
-
Product Palonosetron Hydrochloride
anti-emesis after chemotherapy or radiotherapybrhrbrPalonosetron, Aloxi -
Product Pegfilgrastim
long lasting filgrastim, pegylated filgrastim, neulasta -
Product PegFilgrastim
a long lasting rhG-CSF, Bulk and injection (PFS): 6mg in 0.6ml -
Product Ranibizumab
Wet Macular Degeneration, API -
Product Recombinant Human Bone Morphogenetic Protein-2
rhBMP-2, a new bone repair material,registered as an implanted medical device, Active pharmaceutical ingredient (API), l Tablet (vial): 1mg/vial
-
Product Recombinant Human Bone Morphogenetic Protein-2
rhBMP-2, a new bone repair material,registered as an implanted medical device, Active pharmaceutical ingredient (API),Tablet (vial): 1mg/vial
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance





















%20-%20copia-comp247805.jpg)








%20(1)%20(1)-comp246058.png)










.png)