Oligonucleotide Synthesis Service and Partnerships
Product Description
Biotage Sweden

-
SE
-
2023On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Biotage Sweden

-
SE
-
2023On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Biotage Sweden (7)
-
Product Bespoke Oligonucleotide Synthesis Service and Partnerships
Whether you need a single sequence for a project or are looking for a long-term collaborative partner, we synthesize and deliver the best DNA/RNA oligonucleotides for your precise needs. Oligonucleotides are the backbone of molecular diagnostics and the future of personalised medicine. We synthesize and d... -
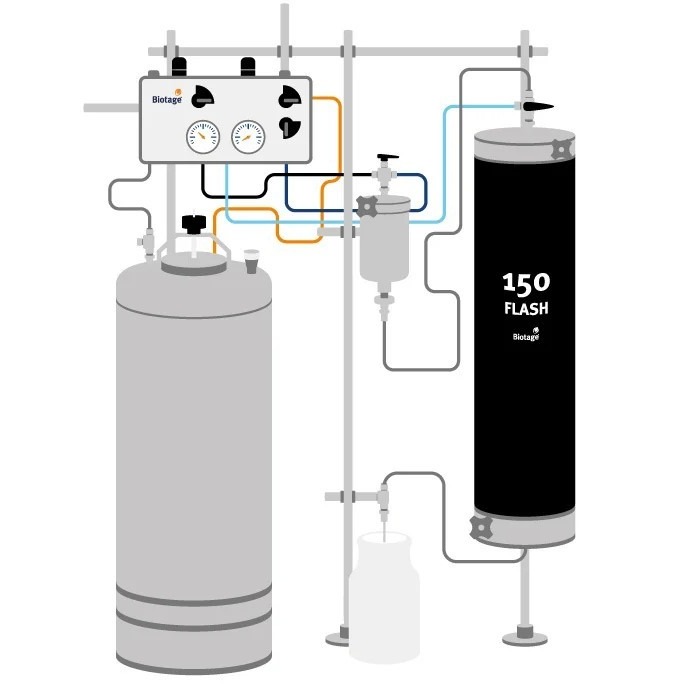
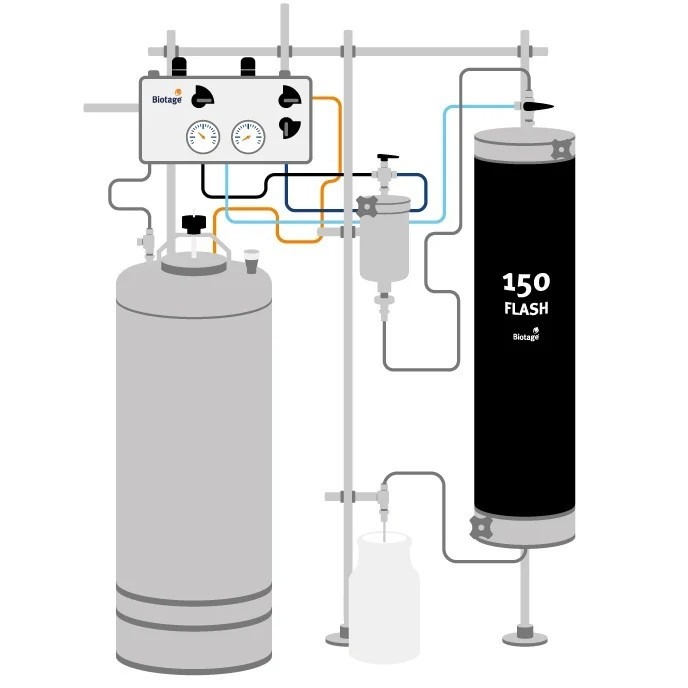
Product Biotage® Flash 150 Chromatography Systems
Batch purification 80% faster than glass columns, 500g samples at 1L /min The Biotage® Flash 150 system supports batch purification up to 80% faster than traditional glass columns. Flash 150 is a simple, robust and reliable system, containing everything needed for large or industrial scale separations. -
Product Biotage® Flash 400 Chromatography Systems
The ultimate purification system, Isolate up to 8 kg of product per run at 6 L/min The Biotage® Flash 400 system is a complete skid-mounted system designed for kilogram scale separations. Built to last and engineered with high quality materials that comply with various cGMP standards. Available in two co... -
Product Biotage® Metal Scavenging Screening Kits
Simple kits help deal with today's impurity challenges and help you to find the best metal scavenger for your application. Biotage® Metal Scavenging Screening Toolkits enable quick and efficient identification of candidate metal scavengers for an application. Containing our most popular metal scavengers,... -
Product Nereus LentiHERO®, a fit-for-purpose solution for lentiviral vector purification
Nereus LentiHERO® shortens the time to process multiple lab-scale lentiviral feedstocks, so reducing bottlenecks for viral vector sample preparation. Nereus LentiHERO® offers the combined benefits of very high recovery of viral particles, significant reduction of contaminants and ability to scale out, unli... -
Product Large Scale Flash Purification Systems
Large scale systems for the purification of target compounds in scale-up studies, in manufacturing and on the pilot plant. Robustness and safety are key factors for any production scale instrument. The Biotage® Flash 75/150/400 and development Biotage® Isolera LS lab-based flash systems need ... -
Product Small Molecule and Synthetic Therapeutic Workflows
Organic Workflow Systems.
Organic chemists synthesizing new molecules follow a general workflow with Synthesis, Purification, Work-Up, and Evaporation steps. We aim to make the whole process faster, simpler and greener.
Peptide Workflow System.
Our tools increase the ...
Biotage Sweden resources (6)
-
News Astrea Bioseparations joins Biotage, enabling ground breaking chromatography solutions
Biotage has completed the acquisition of Astrea Bioseparations, a high-growth chromatography solutions provider from Gamma Biosciences, a life sciences tools platform created by KKR. -
Brochure Large Scale Flash Purification
Large Scale Flash Purification - Biotage flash systems support purification from R+D through to large scale. -
Brochure Biotage Metal Scavengers
Biotage metal scavengers are clean, simple and effective. They help to reduce metal contamination by selectively removing metals from APIs and other pharma comp -
Brochure Custom Oligonucleotide Synthesis
Complex Bespoke DNA/RNA Oligonucleotides for your Diagnostic and Therapeutic Needs -
Brochure Nereus LentiHERO®
Lentiviral Vector Purification - Novel tools to reduce bottlenecks in viral vector processing -
Brochure Accelerate Your Science!
Biotage Small Molecule and Synthetic Therapeutics Workflow Solutions
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




























































.jpg)