Oligonucleotide CRDMO Platform

Product Description
WuXi STA

-
US
-
2015On CPHI since
-
3Certificates
-
5000+Employees
Company types
Categories
WuXi STA

-
US
-
2015On CPHI since
-
3Certificates
-
5000+Employees
Company types
More Products from WuXi STA (13)
-
Product F2CS - "Fast to Clinical Supply" Platform
Your innovative therapy is ready to reach a key milestone with the IND date quickly approaching. You need a reliable partner to formulate and manufacture your therapy to meet the IND filing requirements and the dose needs for phase I studies fast.
Every innovation is unique so your partner needs... -
Product F4CL - "Fast for Commercial Launch" Platform
When your CMC projects approach the final step to commercial approval, you need to consider how to be sure your drugs successfully reach your intended markets.
Regulatory agencies have different requirements and timing. Trust in the right partner so you don’t need to worry abou... -
Product IND Enabling Preformulation Package (IEPP)
WuXi STA's IND Enabling Pre-formulation Package (IEPP) is an integrated and end-to-end developability assessment service for new chemical entities (NCE's). Through this comprehensive service, you can efficiently identify challenges and tailored solutions earlier for higher project success rates and lower o... -
Product Small Molecule API Development & Manufacturing
WuXI's unrivaled API development platform provides our client with a robust platform to bring their drug to market. With a proven track record of success and trust in bringing life changing therapeutics to market, we are a go-to partner for clients looking for tailored and customized drug development and m... -
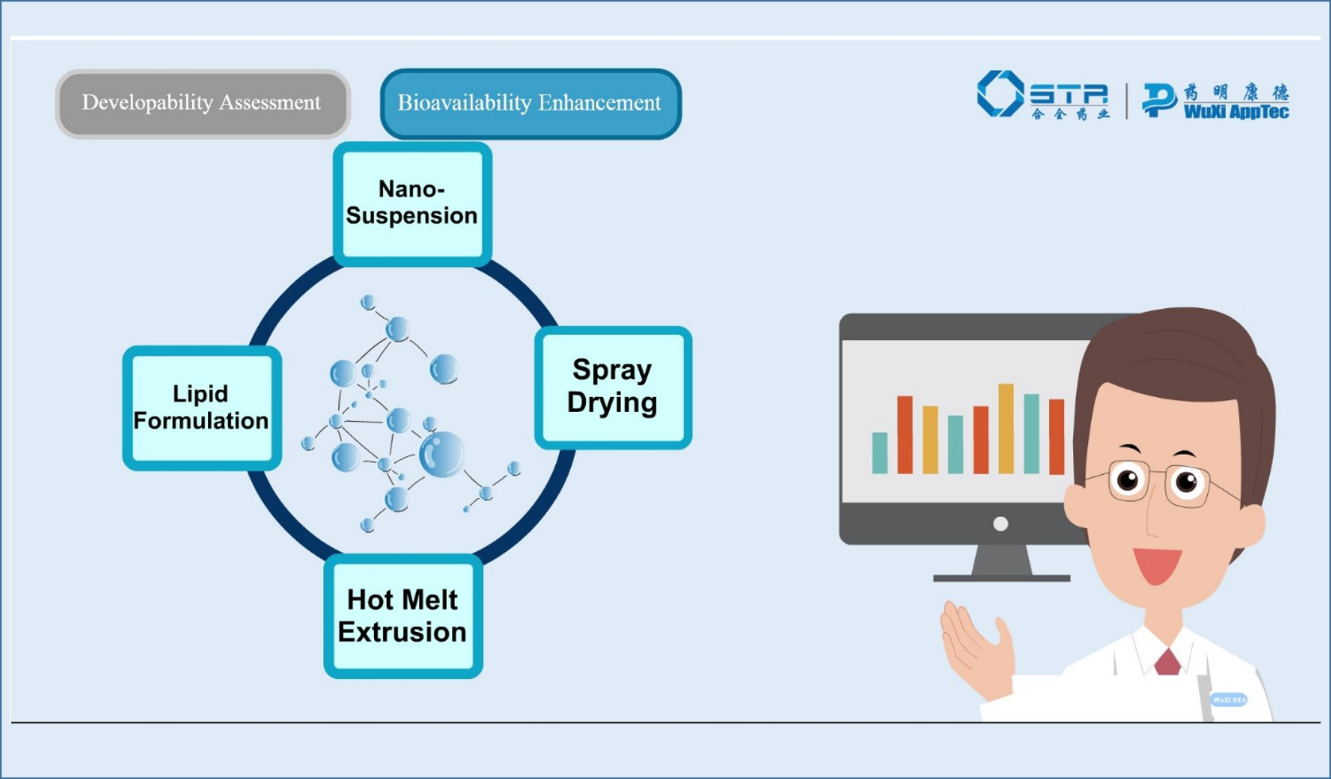
Product Bioavailability Enhancement & Advanced Delivery Technologies
Our bioavailability toolbox offers a customizable solution to your drug delivery challenges.
- Spray-Dried Dispersion (SDD)
- Hot Melt Extrusion (HME)
- Nano-suspensions & Lipid Nanoparticles
- Softgel & Liquid Filled Capsules
- Injectable Formulations\
-
Product Peptide CRDMO Platform
WuXi TIDES's Peptide platform includes a complete offering of services to bring your protein therapeutic to market faster while supporting a wide variety of conjugates including PPMO, Peptide-Antibody, Peptide-Linkers.
Services include but are not limited to: - Comprehensive Discovery Service... -
Product Highly Potent API (HPAPI), ADC Payload, and Payload-Linker Manufacturing
Our Jinshan and Changzhou sites are equipped with multiple R&D labs as well as a dedicated cGMP HP Kilo lab and plant for producing and manufacturing highly potent APIs (HPAPI's), antibody drug conjugates (ADC's), payloads, and payload-linkers. We currently offer drug product manufacturing at STA’... -
Product Analytical Development Services
Our comprehensive suite of analytical services help to supplement the development and manufacture of your product, ensuring high quality and consistency throughout the process.
Services include:
- Analytical method development and validation
- API and drug product release tes... -
Product in-vivo PK Studies
Our drug product pre-formulation and formulation development services can also includes in vivo PK studies, conducted with multiple animal species and a rapid turnaround time. This additional service further extends our full suite offering to our clients while eliminating the need for additional vendor sou... -
Product Injectable Formulation Development & Fill-Finish Services
WuXi STA offers a wide range of tailored services and solutions for clients developing injectable formulations. Form types include solution, emulsion, sterile powder (lyophilized) and liposome. Our offering includes formulation development, performance analysis and characterization testing, and fill/... -
Product Global Regulatory Affairs & CMC Submission Support
WuXi STA's dedicated Global Regulatory Affairs CMC teams offers comprehensive CMC documentation support for IND, CTA, MAA, and NDA applications in western countries and China. Our highly experienced team works with your NCE programs to prepare Module 2 and Module 3 of your regulatory dossier through foreca... -
Product Packaging & Labeling Solutions
At WuXi STA, we understand the need for flexibility at the early stages of clinical testing and how aspects such as effective dose, stability and compliance requirements can change your needs abruptly. Our packaging team will work with you to collect the relevant data and test various packaging options as ...
WuXi STA resources (16)
-
News New drug product service delivers expedited CTM
Biotech and pharma innovators are facing considerable challenges to find CDMO partners with capacity and capability to quickly develop and deliver high-quality CTM with flexible options+ -
Sponsored Content End game strategies to reach patients globally and swiftly
The journey for new therapies is long; wondering if a proven therapy will be produced and distributed efficiently to reach the intended markets as soon as possible should not be another burden -
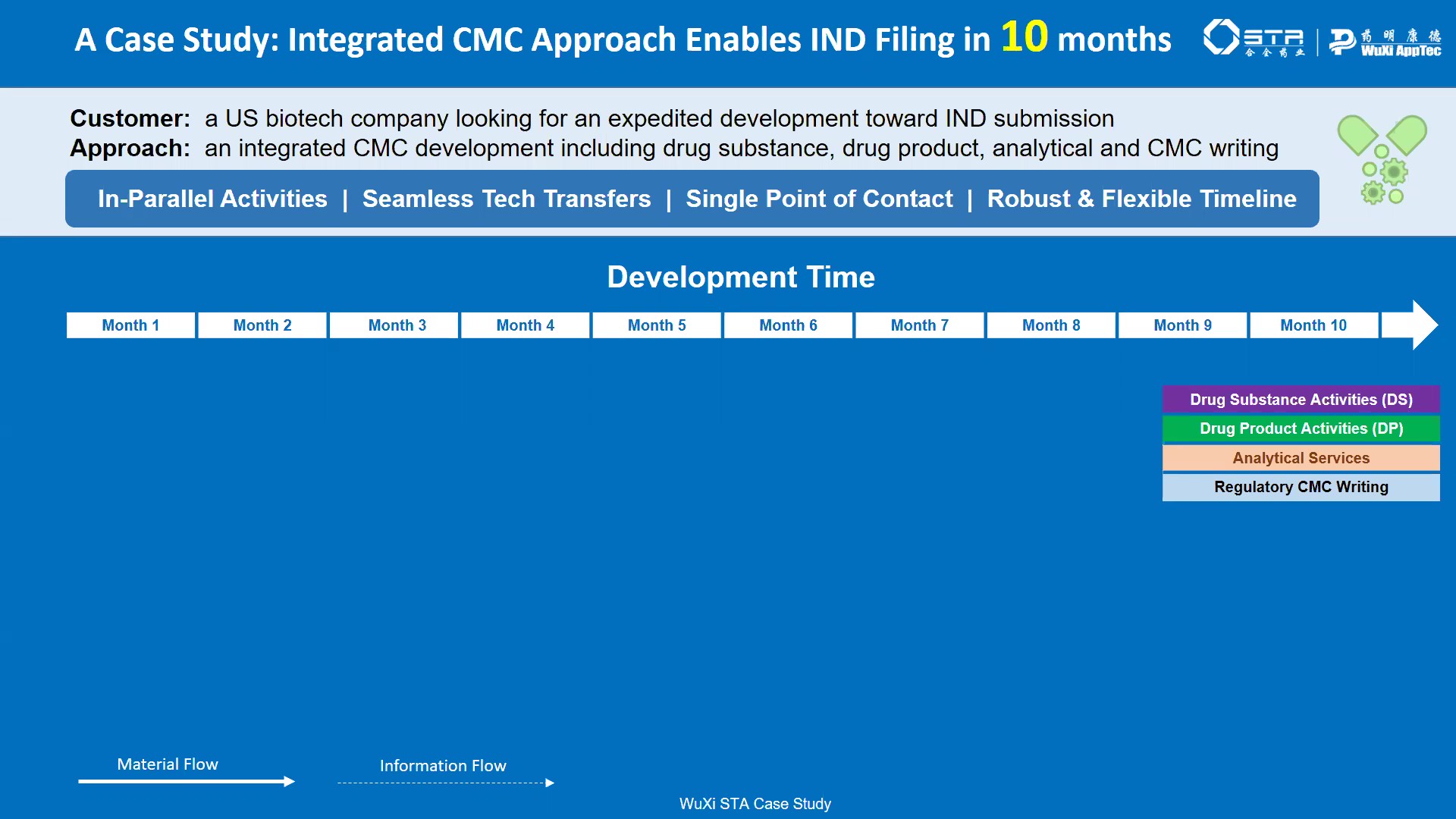
Video Case Study - Integrated CMC to IND in 10 Months
A Case Study of WuXi Speed and Service for a client. The project, which encompassed an integrated CMC approach to bring a molecule to IND phase was completed in 10 months -
News Hoth Therapeutics and WuXi strike API and drug product deal for cancer therapeutic
WuXi will use its new modality platform to provide an end-to-end solution for oligonucleotide peptide -
Video WuXi STA IND Enabling Preformulation Package
WuXi STA's IND Enabling Preformulation Package (IEPP) is an integrated developability assessment service for new chemical entities (NCEs) to efficiently identify challenges and solutions earlier for higher success rates and lower overall costs. This video explains the package and process. -
News WuXi STA completes acquisition of its first European facility
The purchase of BMS's Couvet, Switzerland facility adds commercial-scale production capacity for capsule and tablet dosage forms -
Brochure F2CS: Fast to Clinical Supply
F2CS - fast to clinical supply service package delivers reliable clinical trial materials (CTM) fast with flexible options custom developed by highly experienced integrated teams equipped with advanced technologies, working in parallel to save time. -
News 2021, WuXi STA Again Wins CMO Leadership Awards in All Six Core Categories
WuXi STA has been deeply involved in the CDMO industry for more than ten years. In the year of 2020 alone, WuXi STA served more than 470 customers worldwide, supporting 13 NDA approvals globally including 5 from US FDA, 4 from EU EMA, 2 from China NMPA and 2 from Japan PMDA. Dr. Minzhang Chen, CEO of WuXi STA, commented: “Thanks to our partners for their recognition of WuXi STA, and we will continue to leverage our end-to-end CMC platform, along with the global standard quality systems to empower more partners to accelerate their pathway to market for the benefit of global patients.” -
Whitepaper IND Enabling Pre-formulation Package (IEPP)
WuXi STA's pre-formulation package IEPP (IND Enabling Pre-formulation Package) helps you select the best drug candidates at late discovery phase and develop the most suitable formulation for phase I in 8-12 weeks,
-
News Three WuXi STA Facilities Pass Pre-Approval Inspection From NMPA
STA Pharmaceutical, a WuXi AppTec company (WuXi STA), a leading contract development and manufacturing organization (CDMO) announces that three of its sites in China have successfully passed pre-approval inspections (PAI) by the China National Medical Products Administration (NMPA) concurrently for an innovative drug from its partner. -
News Three WuXi STA facilities pass pre-approval inspection from NMPA
The achievement validates the company's CMC capabilities and quality -
News WuXi STA Opens Oligonucleotide Large-Scale Manufacturing Facility
STA Pharmaceutical Co., Ltd., (WuXi STA) – a subsidiary of WuXi AppTec – today announced the opening of its large-scale oligonucleotide oligonucleotide active pharmaceutical ingredient (API) manufacturing facility in Changzhou, China. This significant milestone marks WuXi STA's establishment of a comprehensive one-stop platform to support the process R&D and manufacture of oligonucleotide APIs from preclinical to commercial. It enables customers around. -
News WuXi STA to Purchase Bristol Myers Squibb Manufacturing Facility in Couvet, Switzerland
Bristol Myers Squibb (NYSE: BMY) and WuXi STA – a subsidiary of WuXi AppTec – today announced that WuXi STA has agreed to purchase Bristol Myers Squibb’s manufacturing facility in Couvet, Switzerland. The Couvet site will be the first facility in Europe for WuXi STA, a leading Contract Development and Manufacturing Organization. The acquisition will enhance WuXi STA’s existing capabilities while growing capacity to support its partners’ life-saving work. -
News WuXi STA to grab foothold in Europe with planned BMS facility purchase
The acquisition will expand the CDMO's capabilities and capacity to serve European markets -
News WuXi Biologics, WuXi STA and Antengene collaborate to advance ADC candidate
The Wuxi companies will provide process development, scale-up, and GMP to advance Antengene's ADC program to clinical stage
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance