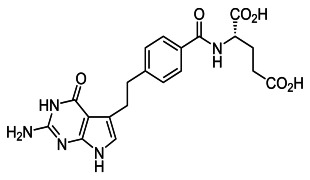
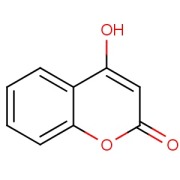
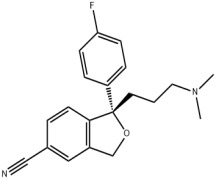
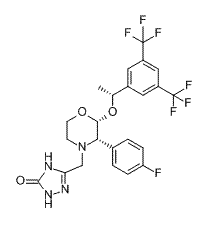
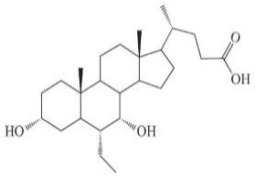
Nomegestrol acetate

Product Description
Aspen Healthcare. We Care

-
NL
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
Specifications
Aspen Healthcare. We Care

-
NL
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Aspen Healthcare. We Care (58)
-
Product Cidofovir
This API is part of the FCC product portfolio -
Product Etelcalcetide
Specific Disclaimer. This active ingredient could be protected by a patent in force and would be therefore developed on request exclusively for the purposes stated in Article 10.6 of the Directive 2001/83/EC as amended by the Directive 2004/27/EC (Bolar provision). No activity will be performed with the ac... -
Product Fluphenazine Decanoate
This API is part of the FCC product portfolio -
Product Fluphenazine Hydrochloride
This API is part of the FCC product portfolio -
Product Levalbuterol Hydrochloride
This API is part of the FCC product portfolio -
Product Methazolamide
This API is part of the FCC product portfolio -
Product Noscapine on Resin
This API is part of the FCC product portfolio -
Product R-Baclofen
This API is part of the FCC product portfolio -
Product Ropivacaine Base
This API is part of the FCC product portfolio -
Product S-Ropivacaine Hydrochloride
This API is part of the FCC product portfolio -
Product Scopolamine Base
This API is part of the FCC product portfolio -
Product Segesterone acetate
Specific Disclaimer: This active ingredient could be protected by a patent in force and would be therefore developed on request exclusively for the purposes stated in Article 10.6 of the Directive 2001/83/EC as amended by the Directive 2004/27/EC (Bolar provision). No activity will be performed with the ac...
Aspen Healthcare. We Care resources (4)
-
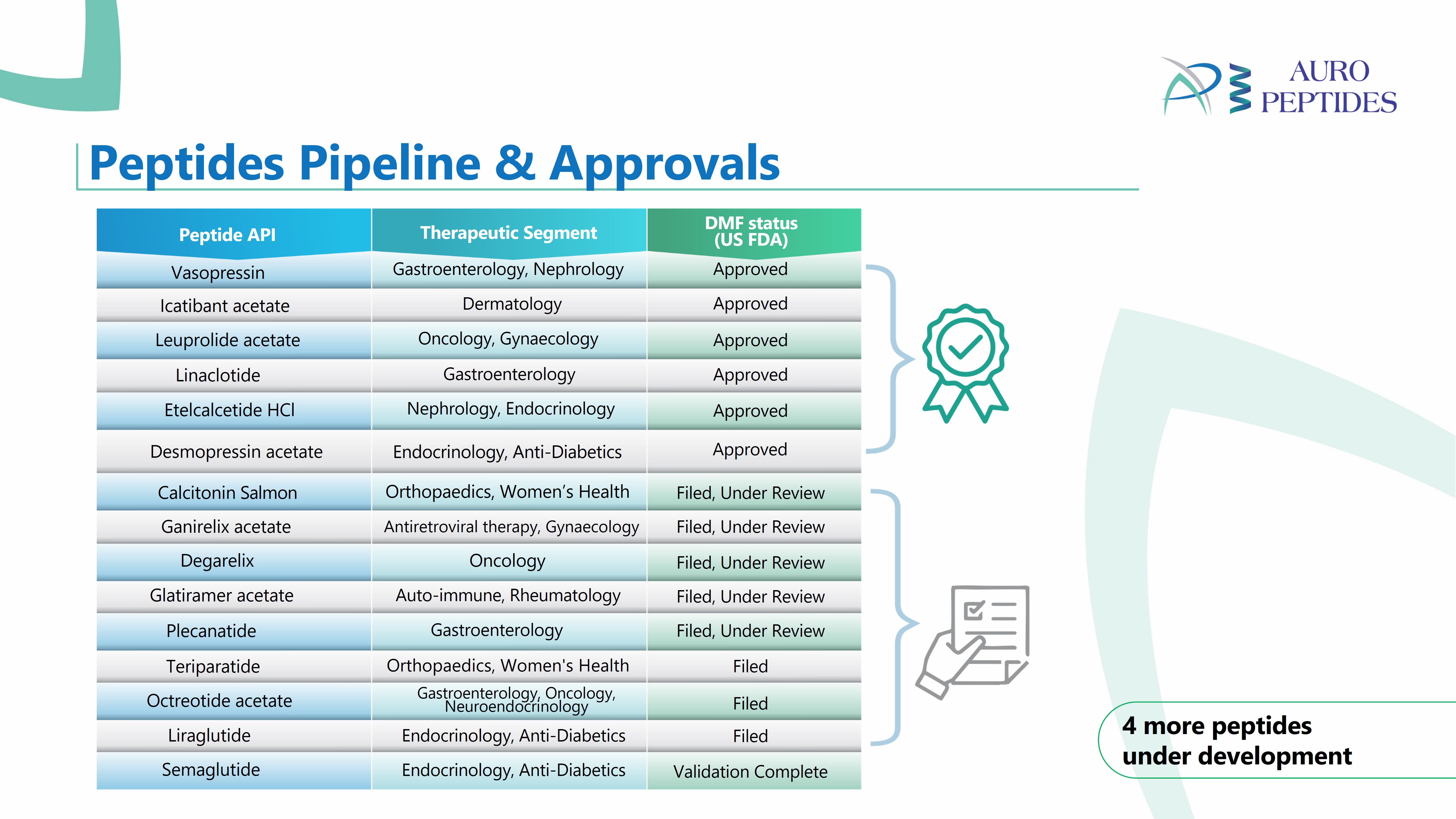
Sponsored Content Aspen API’s Green answer to peptide synthesis
Our Green Continuous Liquid Phase Peptide Synthesis (GC-LPPS) combines the advantages of the classical solution-phase synthesis with the solid-phase approach. -
Brochure Aspen. Healthcare. We Care.
Aspen is a global specialty and branded pharmaceutical company committed to promoting access to medicines and improving the health of patients across the world through our high quality, affordable products. Active at every stage of the value chain, we are uniquely diversified by geography, product and manufacturing capability. -
News CPHI Trend Report - Sustainability in Pharma
While in the past, much of the discussion around sustainability in the pharmaceutical industry rightly focused on efforts to minimize the environmental impact of drug production, the needle has now shifted. There are signs that companies not only understand that it represents one of the world’s biggest challenges moving forward, but also that they are starting to incorporate sustainability practices into a much broader suite of operations. -
News Pharmaceutical shortages open up debate on reshoring of API manufacturing
Panel at CPHI Festival of Pharma discusses options for companies to ensure robust supply chains and reduce overseas supply dependency
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance