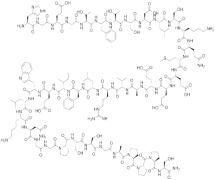
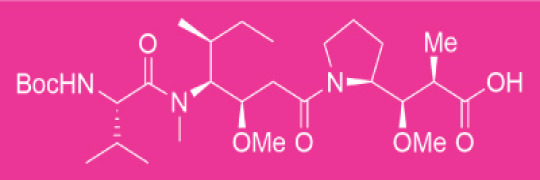
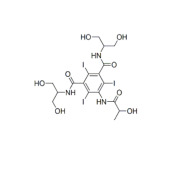
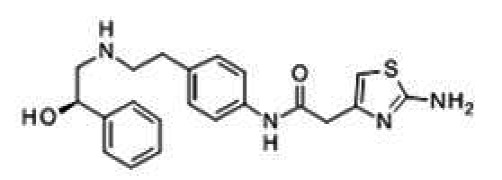
No heat generation

Product Description
Microchem Srl.

-
IT
-
2015On CPHI since
Microchem Srl.

-
IT
-
2015On CPHI since
More Products from Microchem Srl. (8)
-
Product Delumping
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes delumping. It can be used successfully not only to reduce the particle size, but also to remove lumps and agglomerates. -
Product Dispensing
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes dispensing. It can be used to dispense and re-pack bulk api into small containers at any amount. -
Product Excellent yield
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes excellent yield. It guarantee to reduce the product build-up and to achieve yields higher than 99% for the industrial process, starting from quantities of 1kg. -
Product Extremely precise psd
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes extremely precise psd. It able to obtain very fine and precise particle size distribution with only a single micronization passage for nearly all pharmaceutical ingredients. -
Product Quality control instruments
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes quality control instruments. It includes malvern mastersizer particle sizer, ft-ir, melting point analyser, uv-vis spectrophotometer, optical microscope with image analysis, etc. -
Product Quality control laboratory activities
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes quality control laboratory activities. It includes identification analysis, cleaning validation study, particle size distribution analysis, microbiological monitoring of environment, water an... -
Product Scalability
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes scalability. It enables the active ingredient to be followed from its first r&d phases through to marketing; it allows us to operate with the same product from batches of 0.2 g up to several ... -
Product Validation activities
Microchem Srl. provides contract micronization of active ingredients for the pharmaceutical industry which includes validation activities. It includes process parameter study, to define and support the validation activity, process validation, analytical method validation, etc.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance