Microbiology Testing

Product Description
SGS Group Management SA

-
CH
-
2020On CPHI since
-
5000+Employees
Company types
Primary activities
Categories
Specifications
SGS Group Management SA

-
CH
-
2020On CPHI since
-
5000+Employees
Company types
Primary activities
More Products from SGS Group Management SA (13)
-
Product Extractables & Leachables
Extractables & leachables studies enable drug sponsors to quantify and identify the risks of potentially toxic leachable impurities migrating into a drug solution from container closure systems, processing equipment or packaging. Regulatory bodies such as the US Food and Drug Administration (FDA) and T... -
Product Quality Control Testing
Bringing a compound from the laboratory to the market is a long and winding road with a great number of scientific, safety and regulatory challenges. SGS has been offering high quality analytical testing and clinical research services to support drug research, registration and production.
SGS leve... -
Product Biosafety Testing
Life-saving medicines are heavily regulated during development, manufacture and distribution. To fulfill regulatory requirements, the biopharmaceutical industry is increasingly looking for independent service providers who can deliver comprehensive characterization solutions on one site.
SGS’s glo... -
Product Viral Safety Testing
SGS’s global center of excellence for cell bank characterization & virus testing is located in the United Kingdom and provides services with ultimate reliability, highest GLP/cGMP quality & scientific expertise.
For any of your biologics, we help you comply with the global regula... -
Product Biologics Charaterization
A full package of Biologics testing services to cGMP standards from early phase characterization including Biosafety to Quality Control release testing enabling you to outsource your biologics. -
Product Impurities and contaminants analysis
Impurities and contaminants analysis in biotechnology products from SGS – a range of services providing impurities and contaminants characterization in biotechnology derived products and microbial contamination control.To help you meet ICH Q6B guidelines, you need accurate impurities and contaminants analy... -
Product Analytical Chemistry Services
Delivering a product from discovery to the market is a long and often difficult process filled with a great number of scientific, safety and regulatory challenges. From early discovery to final product release, SGS offers a broad portfolio of high quality, analytical chemistry services using advanced metho... -
Product Stability Testing
From study design, storage and shelf-life stability to monitoring, analytical testing and documentation, SGS offers comprehensive stability testing services.
When it comes to testing the stability of your pharmaceutical and biologic projects, you need world-class expertise and resources. With over 2... -
Product Package and Container Testing
SGS realizes that the package or container that carries your product is vital to its safety and efficacy: a weak container, or one that allows contamination of your product, could put the health of your customers and your reputation at risk. SGS package and container testing services offer a ran... -
Product Cell bank and virus seed characterization
As a world leader in biopharmaceutical testing services, we offer you a comprehensive range of biologics safety testing , including virology, cell and molecular biology, as well as microbiology and electron microscopy. As a result, we can help you to ensure product safety and meet your regulatory requireme... -
Product Bioanalysis Testing
SGS has the expertise to both develop assays from scratch (including LC-MS/MS, immunoassays and cell-based assays) and to support large scale routine sample analyses, from regulatory pre-clinical (toxicology) to early and late clinical studies (Phase I to IV). -
Product CDMO Services
CMC CONSULTANCY PRE-CLINICAL FORMULATION SCREENING FORMULATION DEVELOPMENT ANALYTICAL SERVICES GMP CLINICAL MANUFACTURING COMMERCIAL & SPECIALS MANUFACTURING CLINICAL TRIAL SUPPLY
SGS Group Management SA resources (13)
-
News SGS Joins Oxford University Consortium Led by the Jenner Institute to Develop COVID-19 Vaccine
In a consortium led by the Jenner Institute, Oxford University, SGS has joined forces with specialists in infectious diseases, research and innovation, and pharmaceuticals to rapidly develop, scale-up and produce a potential vaccine called ChAdOx1 nCov-19 -
Brochure Analytical Testing and Quality Control Solutions
Bringing a compound from the laboratory to the market is a long and winding road with a great number of scientific, safety and regulatory challenges. SGS has been offering high quality analytical testing and clinical research services to support drug research, registration and production.
SGS leverages its conveniently located network of laboratories and clinical trial facilities present in North America, Europe and Asia-Pacific, to deliver harmonized solutions to large pharmaceutical and biotechnology firms.
Our experts provide effective and efficient testing solutions for analytical development, biologics characterization, biosafety and quality control, as well as clinical research services. We perform a variety of tests that are bespoke, client-specific and support the full clinical development, from Phase I First-in-Human trials in our Clinical Pharmacology Units to Phase II and Phase III studies in patients. -
News SGS Signs Analytical Testing Agreement with AstraZeneca for COVID-19 Vaccine Candidate
SGS is pleased to announce the signing of an agreement with AstraZeneca for the provision of safety, quality control and analytical testing services in support of AZD1222 – a COVID-19 vaccine candidate co-invented by the University of Oxford and its spin-out company, Vaccitech and licensed by AstraZeneca. -
Brochure Nitrosamine Impurities Testing Solutions
SGS Life Sciences has considerable expertise in the method development of nitrosamine determination in pharmaceutical products. SGS has established a specific method for NDMA which can be applied to various different matrices. Alternatively, a platform method, based on trace-level detection by LC-MSMS, is also available and provides rapid and simultaneous determination of up to ten different, targeted nitrosamines. Although with more limited application, the SGS network is also able to support specialist methodologies such as GC-MSMS. Our experience in optimizing extraction procedures allows application of these methods to drug products, API’s, and raw materials.
-
News Managing Your Life Sciences Quality Management System Remotely
Telecommuting and online commerce has been with us for some time and works seamlessly in many industries, but the current state of events has forced companies to apply remote management to other areas that have historically been considered "hands-on" job functions. -
Brochure Biologics Testing Services
A full package of Biologics testing services to cGMP standards from early phase characterisation including Biosafety to Quality Control release testing enabling you to outsource your biologics. -
News Vaccine Testing to Ensure Successful and Faster Vaccine Research and Development
Following its emergence earlier this year, the novel coronavirus (SARS-CoV-2) has spread rapidly and snowballed into a pandemic. Everyone – citizens, businesses, and governments – yearns for life to go back to normal soon. -
Brochure Analytical Chemistry Services
Delivering a product from discovery to the market is a long and often difficult process filled with a great number of scientific, safety and regulatory challenges. From early discovery to final product release, SGS offers a broad portfolio of high quality, analytical chemistry services using advanced methods and instrumentation.
SGS leverages its conveniently located network of laboratories present in North America, Europe and Asia-Pacific, to deliver harmonized solutions to local markets as well as international pharmaceutical and biotechnology firms.
Our recognized experts assist in providing effective and efficient solutions for R&D, analytical development, and quality control testing, helping to expedite the molecule to market.
-
Brochure Bioanalysis Testing Solutions
SGS has the expertise to both develop assays from scratch (including LC-MS/MS, immunoassays and cell-based assays) and to support large scale routine sample analyses, from regulatory pre-clinical (toxicology) to early and late clinical studies (Phase I to IV). CONTINUOUS -
Brochure Biosafety Testing Solutions
Life-saving medicines are heavily regulated during development, manufacture and distribution. To fulfill regulatory requirements, the biopharmaceutical industry is increasingly looking for independent service providers who can deliver comprehensive characterization solutions on one site.
SGS’s global center of excellence for cell bank characterization & virus testing is located in the United Kingdom and provides services with ultimate reliability, highest GLP/cGMP quality & scientific expertise.
As trailblazers in the development of the biosafety testing industry, our team in Glasgow have developed and validated novel nucleic acid technologies, such as real-time PCR, RAPD, Sequencing, Non
radioactive Southern Blotting, Next Generation Sequencing (NGS). For any of your biologics, we help you comply with the global regulatory guidelines and testing requirements.
-
Video VIDEO: SGS vs COVID-19: Biologics Characterization Services
SGS' integrated network of laboratories provides a wide range of essential services to support the fight against COVID-19. We provide established expertise and resources for all aspects of biopharmaceuticals characterization, from physico-chemical properties, to primary, secondary, and tertiary structures, as well as aggregation.
For more information, contact lss.info@sgs.com or visit: https://www.sgs.com/lifescience. -
Video VIDEO: Extractables & Leachables Studies
Watch to find out more about how our Extractables & Leachables studies enable drug sponsors to quantify and identify the risks of potentially toxic leachable impurities migrating into a drug solution from container closure systems, processing equipment or packaging, to ensure patient safety and drug product efficacy.
Visit https://www.sgs.com/en/life-sciences/ to find out more. -
Video VIDEO: Nitrosamine Impurities Testing Services
The presence of the #nitrosamine N-nitrosodimethylamine (#NDMA), in certain sartan APIs has resulted in several regulatory warnings and recalls of contaminated products. We have considerable expertise in the method development for detecting the presence of NDMA and other nitrosamines to help you to ensure product safety and comply with USFDA and EMA requirements.
For more information, contact lss.info@sgs.com or visit: https://www.sgs.com/lifescience.
#Nitrosamine #DrugSafety #DrugDevelopment
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

.jpg)
















.jpg)

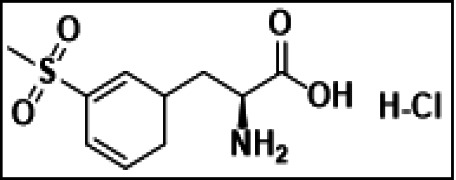


















![Heptanoic acid,4-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-3-hydroxy-6-m...](/46/product/123/13/23/p0th_S.jpg)