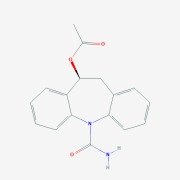
Metoprolol Succinate

Product Description
CTX LifeSciences Pvt. Ltd.

-
IN
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
Specifications
CTX LifeSciences Pvt. Ltd.

-
IN
-
2015On CPHI since
-
4Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from CTX LifeSciences Pvt. Ltd. (29)
-
Product Ticagrelor
CTX Manufacturer Form - II
O DMF available.
CEP Approved.
CTX Lifesciences respects patent laws and conventions of pharmaceuticals as applicable in different countries.
API/Substances covered by patent are not offered to the countrie... -
Product Brexpiprazole
Brexpiprazole API is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer’s disease. It is an atypical antipsychotic. -
Product Saxagliptin
Saxagliptin is used along with diet and exercise to lower blood sugar levels in patients with type 2 diabetes (condition in which blood sugar is too high because the body does not produce or use insulin normally). Saxagliptin is in a class of medications called dipeptidyl peptidase-4 (dpp-4) inhibitors. -
Product Vildagliptin
Vildagliptin is an oral anti-hyperglycemic agent (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of Glucagon-like peptide-1(GLP-1) and Glucose-dependent insulinotropic polypeptide(GIP) by DPP-4, allowing GLP-1 and GIP to potentiat... -
Product Hydrochlorothiazide
Hydrochlorothiazide is a benzothiadiazine that is 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxidesubstituted by a chloro group at position 6 and a sulfonamide at 7. It is diuretic used for the treatment of hypertension and congestive heart failure. It has a role as a xenobiotic, ... -
Product Oxcarbazepine
Oxcarbazepine is a keto analogue of Carbamazepine and, like the parent drug, is a potent anticonvulsant used alone or in combination with other agents in the therapy of poartial seizures. Oxcarbazepine is a dibenzazepine carboxamide derivative with an anticonvulsant property. As a prodrug, oxcarbazepi... -
Product Rosuvastatin Calcium
Rosuvastatin Calcium is the calcium salt form of rosuvastatin, a statin with antilipidemic activity. Rosuvastatin selectively and competitively binds to and inhibits hepatic hydroxymethyl-glutaryl coenzyme A (HMG-CoA) reductase, the enzyme which catalyzes the conversion of HMG... -
Product Eslicarbazepine acetate
Eslicarbazepine acetate is the acetate ester, with S configuration, of licarbazepine. An anticonvulsant, it is approved for use in Europe and the United States as an adjunctive therapy for epilepsy. It has a role as an anticonvulsant. It is an acetate ester, a dibenzoazepine, a carboxamide and a ... -
Product Deferasirox
Deferasirox is a synthetic, orally bioavailable, achiral, tridentate triazole derived from salicylic acid with iron-chelating activity. Deferasirox chelates iron at a 2:1 (ligand:iron) ratio. Because of its oral availability and long plasma half-life, this agent may be superior to&... -
Product 4-Amino-6-chlorobenzene-1,3-disulfonamide (CADS)
CTX offers a wide range of intermediates which includes 5-chloroaniline-2,4-disulfonamide (CADS). API- Hydrochlorothiazide. Contact us for more information. -
Product Acetazolamide
Acetazolamide is a sulfonamide derivative with diuretic, antiglaucoma, and anticonvulsant properties. Acetazolamide is a non-competitive inhibitor of carbonic anhydrase, an enzyme found in cells in the proximal tube of the kidney, the eye, and glial cells. Inhibition of this enzyme in the kidney prevents e... -
Product Amiodarone Hydrochloride
Amiodarone Hydrochloride is the hydrochloride salt of an iodine-rich benzofuran derivative with antiarrhythmic and vasodilatory activities. As a class III antiarrhythmic agent, amiodarone blocks the myocardial calcium, potassium and sodium channels in cardi...
CTX LifeSciences Pvt. Ltd. resources (1)
-
Video CTX Lifesciences - Your Preferred API, Intermediates Manufacturing & CDMO Partner from India.
CTX Lifesciences, a vertically integrated Active Pharmaceutical Ingredient (API ) and intermediates manufacturing company supplying & exporting its products across 87 countries. It was established in the year of 2004 in Surat, India and is a part of family owned $600 Million group. This Facility is having over 1000 KL reaction volume and is spread across 38 acres land with sufficient area for expansion. The Active Pharmaceutical Ingredient (API ) products manufactured are backward integrated into intermediates and Key Starting Materials( KSM ), allowing better controls on quality of the products and supply reliability for our customers. This facility is compliant and approved by USFDA, EUGMP, Health Canada, ANVISA, RUGMP, KFDA, COFEPRIS & Iran MOH. The facility is having excellent EHS Management capabilities and certified with ISO 14001:2015 , ISO 45001:2018. This facility is also Ecovadis Rated and Pharmaceutical Supply Chain Initiative ( PSCI ) Audited. Driven...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance