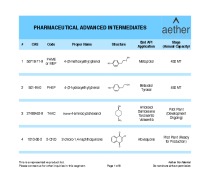
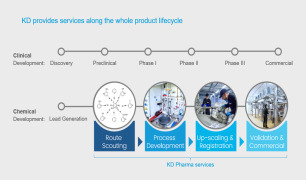
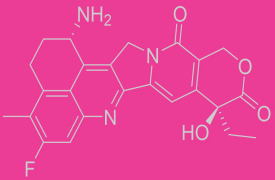
Method verifications/transfer

Product Description
Syngene International Ltd.

-
IN
-
2015On CPHI since
-
5000+Employees
Company types
Syngene International Ltd.

-
IN
-
2015On CPHI since
-
5000+Employees
Company types
More Products from Syngene International Ltd. (65)
-
Product Identification & characterization of impurities
Syngene International Ltd. provides wide range of services which includes identification & characterization of impurities. It belongs to analytical/stability services category. It can be executed with preparatory HPLC using various detectors such as UV/ELSD/MS or supercritical fluid preparatory chromatogra... -
Product In-vivo Pharmacology
Syngene International Ltd. provides wide range of services which includes in-vivo pharmacology. It belongs to biology services category. It includes : oncology, metabolic disorders, inflammation, pain models, auto-immune disorders osteoarthritis, hypersensitivity, others. -
Product library synthesis
Syngene International Ltd. provides wide range of services which includes library synthesis. It belongs to chemistry services category. It includes delivered many libraries with the library size varying from 50 to 3000, delivered libraries comprising of simple (2 - 5 steps) to more complex (8 - 12) steps t... -
Product materials chemistry
Syngene International Ltd. provides wide range of services which includes materials chemistry. It belongs to chemistry services category. It offers: integrate all types of data to build a high quality sar, scaffold hopping and fragment based, design services to get to new leads, target focused molecule des... -
Product Medicinal chemistry
Syngene International Ltd. provides wide range of services which includes medicinal chemistry. It belongs to chemistry services category. It includes hit-to-dead / lead optimization: It starts from the hit(s) which are provided by client or taken from prior-art, analyze the hit compounds to understand the ... -
Product Method development/validation
Syngene International Ltd. provides wide range of services which includes method development/validation. It belongs to analytical/stability services category. It includes analytical method development: purity analysis by hplc, uplc and gc, reaction monitoring using uplc-ms and gc-ms, quantitative nmr analy... -
Product Molecular Biology
Syngene International Ltd. provides wide range of services which includes molecular biology. It belongs to biology services category. It includes recombinant dna engineering, gene library construction & screening, strain engineering, gene expression analysis. Contact us for more information. -
Product Phys-chem studies/5 batch analysis
Syngene International Ltd. provides wide range of services which includes phys-chem studies/5 batch analysis. It belongs to analytical/stability services category. It includes method development/method transfer, non glp screening and impurity profiling by gradient hplc or gc, method validation. -
Product polymer characterization
Syngene International Ltd. provides wide range of services which includes polymer characterization. It belongs to polymer research services category. It is a well-equipped with analytical instruments such as GPC, DSC, FTIR, viscometry, brookfield viscometer, and NMR to analyse different kinds of polymers. ... -
Product polymerization process development (gmp/non-gmp)
Syngene International Ltd. provides wide range of services which includes polymerization process development (gmp/non-gmp). It belongs to polymer research services category. It includes handling high pressure reactors to conduct polymerization reactions which includes hydrogenation of polymers in batch rea... -
Product Polymorph and salt screening
Syngene International Ltd. provides wide range of services which includes polymorph and salt screening, It belongs to process R&D and cGMP manufacturing services category. It identify the various forms possible for a substance, thermodynamically most stable form and the process to prepare the most stable f... -
Product Pre-formulation
Syngene International Ltd. provides wide range of services which includes pre-formulation. It belongs to formulation development services category. It includes physico-chemical characterization / powder characteristics / drug excipient compatibility study : ph- solubility profile and pka determination, sol...
Syngene International Ltd. resources (24)
-
News Power of AI to identify better drug targets and drug candidates
AI helps identify better targets and candidates for drug development faster by utilising large datasets of biological and chemical information and applying machine learning algorithms to analyse the data. -
News Q1 - FY24 - Highlights
Syngene revenue up 26% to Rs. 832 crores, PAT up 26% to Rs.93 crores in the first quarter (1) Acquisition of biologics manufacturing facility in Bangalore to complete by the end of the third quarter in FY 24
(2) US FDA approval for API manufacturing plant in Mangalore received
(3) Acquisition of land in Hyderabad to support long term growth in research services -
News Our client Panbela Announces Issuance of New Patent in Australia
MINNEAPOLIS (GLOBE NEWSWIRE) July 17, 2023, Panbela Therapeutics, Inc. (Nasdaq: PBLA), a clinical-stage biopharmaceutical company developing disruptive therapeutics for the treatment of patients with urgent unmet medical needs, announced an Issue Notification for the Australian patent 2019213664 titled “METHODS FOR PRODUCING (6S,15S)-3,8,13,18- TETRAAZAICOSANE-6,15-DIOL”. This patent, developed in collaboration with Syngene International Ltd., an integrated research, development, and manufacturing services company, claims a novel process with a reduced number of synthetic steps from seventeen to six to produce SBP-101, a lead investigational product. The patent is valid until 2039. -
Whitepaper Regulatory roles of Biophysics CMS
In this Point of View, we discuss the role of biophysicsdriven analytical tools in accurately identifying biologics CQAs and analyzing HOS – resulting in the final product being efficacious and safe for patients from a regulatory perspective -
News Syngene to acquire multi-modal facility from Stelis Biopharma Ltd
Bangalore, July 04, 2023: Syngene (or the “Company”) today announced the acquisition of Unit 3 biologics manufacturing facility in Bangalore, India, from Stelis Biopharma Limited (SBL). The companies have entered into a binding term sheet and, on completion of the transaction, the site will add 20,000 liters of installed biologics drug substance manufacturing capacity for Syngene. The site has the potential for future expansion up to a further 20,000 liters of biologics drug substance manufacturing capacity. It also includes a commercial scale, high speed, fill-finish unit – an essential capability for drug product manufacturing. -
Brochure Integrated Or Standalone?
Read this white paper to gain insights about perceptions around integrated and standalone outsourcing. The white paper is based on surveys conducted across emerging biopharma, mid-size and large pharma in North America, Europe and other regions. -
News Q3-FY23 results-highlights
Bangalore, January 23, 2023: Syngene International Limited announced its third quarter results. Quarterly revenue was up to Rs. 803 crores, while profit after tax for the quarter increased to Rs 110 crores. -
Video Syngene - Large Molecule Bioanalytical Laboratory
Syngene's Large Molecule Bio Analytical Laboratory is India’s premier GLP certified and GCLP compliant lab specializes in immunogenicity and pharmacokinetic (PK) analysis of biologics and biosimilars. The laboratory has extensive experience in working with monoclonal antibodies, recombinant proteins, enzymes, biomarkers, cytokines, vaccines and growth hormones during the early and late phases of the drug development (preclinical and clinical) process. We are having 14+ years of extensive experience in working across a wide variety of technologies and therapeutic areas. The state-of-the-art infrastructure comprises of well-equipped labs & world-class equipment, talented scientific teams, excellent regulatory track record, effective sample management processes, accuracy, data integrity, safety and quality. Read more at https://bit.ly/3fLCwLf -
News Syngene signs 10-year biologics manufacturing agreement with Zoetis
Bangalore, 14 July 2022, Syngene International Limited announced the signing of a 10- year agreement with leading animal health company, Zoetis, to manufacture the drug substance for Librela® (bedinvetmab), a first in class monoclonal antibody used for treating osteoarthritis in dogs. Launched in Europe, the UK and Switzerland, the product won ‘Best new companion animal product’ by IHS Markit Connect in 2021 for its transformational impact on pain relief for canines suffering from this debilitating condition. -
Whitepaper Accelerating PROTAC programs to drug the “undruggable”
In this viewpoint, we discuss how Syngene can help you move your PROTAC programs from hit to lead to the clinic with speed. -
Whitepaper Detection and quantitation of process-related impurities in biopharma manufacturing
In this viewpoint, we discuss the challenges in the detection and quantitation of process-related impurities including nitrosamine impurities and Syngene's capabilities and solutions to address them. -
Whitepaper Intensified Biomanufacturing
In this viewpoint, we discuss how to use technology to enhance productivity using a high seeding density (HSD) approach. The intensified process through converting the traditional fed-batch process to the HSD process could be the answer to increased upstream productivity. -
Video Syngene's Sterile fill finish facility
Syngene’s Sterile Fill-Finish facility is custom-built to meet market demand for small-scale, sterile drug products having short development timelines. Our facility offers Drug substance and Drug product development and manufacturing for both large and small molecules. -
Brochure Syngene -Analytical Development
Syngene’s Analytical Development team is a leader in method development, method validation, method transfer, and application of analytical expertise to ensure the delivery of quality drug products for early as well as late-phase drug development programs. The team has expertise in developing phase-appropriate methods, validation, and transfer of the methods for drug substance and drug product (solid, semisolid, solutions, simple and complex long-acting parenteral formulations). Our team analyzes small molecules (RSMs, intermediates, and APIs), semi-large molecules (polymers, oligonucleotides), and biologics using a variety of spectroscopic, chromatographic, and physiochemical techniques.
-
Video Life at Syngene - Spotlight on Chemical Development - Part 2 with Jayadeva Sajankila
In continuation of our earlier video on ‘Life at Syngene,’ learn from our Research Director, Jayadeva Sajankila, Syngene’s unique capabilities in Solid-state chemistry and how Safety is at the heart of everything we do – personally and professionally.
Visit us at www.syngeneintl.com -
Video Life at Syngene - Spotlight on Chemical Development Part 1 - with Jayadeva Sajankila
Watch this video on what our Research Director, Jayadeva Sajankila, has to say on what makes Syngene unique, new expectations driving drug development today, and more. Visit us at www.syngeneintl.com -
Video Syngene Mangalore Manufacturing Plant - MSEZ
Enabling quality-driven large-scale API manufacturing. State-of-the-art multi-product, multi-client contract manufacturing facility located at Mangalore Special Economic Zone (MSEZ) spread across a sprawling 46 acres campus. Plant designed to support large-scale API and advanced intermediate manufacturing. Offers large scale manufacturing capability in the range of 2 KL to 12.5 KL compliant with ICH Q7 (cGMP) guidelines. -For more information, contact us at bdc@syngeneintl.com -Visit us at www.syngeneintl.com -
Video Syngene Corporate Video 2023
Syngene International Ltd. is an integrated research, development, and manufacturing services company serving the global pharmaceutical, biotechnology, nutrition, animal health, consumer goods, and specialty chemical sectors.Syngene's more than 6000 scientists offer both skills and the capacity to deliver great science, robust data security, and quality manufacturing, at speed, to improve time-to-market and lower the cost of innovation. With a combination of dedicated research facilities for Amgen, Baxter, and Bristol-Myers Squibb as well as 2 Mn sq. ft of specialist discovery, development and manufacturing facilities, Syngene works with biotech companies pursuing leading-edge science as well as multinationals, including GSK, Zoetis and Merck KGaA.#ContractResearch #CRO #CDMO #CMO #Syngene #Biopharma -
Brochure Syngene - Clinical Development
Syngene’s clinical development services team enables early-phase to late-phase clinical trials required for drug development programs across a wide range of therapeutic areas.
Our services include clinical trial management, pharmacokinetic (PK) analysis and bioanalytical studies of small and large molecules, biometrics and clinical data management, and Syngene’s central lab services. We also provide regulatory services for all stages of drug development, medical monitoring services, pharmacovigilance solutions, and medical writing for clinical study documents.
-
Brochure Syngene - Formulation Development
Syngene offers formulation development services to help clients determine optimal dosage levels for therapeutic formulations in oral solid, liquid, and injectable forms. Our integrated services extend to new chemical entities (NCE), late-phase product development, and over-the-counter products with a focus on quality, speed, and cost-efficiency.Our strong scientific team has broad experience and expertise in collaborative practices with a global customer base for formulation development services and analytical support.We cater to pharmaceutical companies worldwide, providing extensive pre-formulation studies, toxicology formulation, formulation development, and analytical method development and validation. Our services include conducting stability studies as per the guidelines of The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and regulatory dossier preparation. -
Brochure Syngene - Chemical Development
Syngene provides comprehensive process research and development services as well as current good manufacturing practices (cGMP) manufacturing capacities to help clients with investigational to commercial-scale development programs. We cater to several industries including pharmaceuticals, specialty chemicals, agrochemicals, polymers, oligonucleotides, animal health, and consumer products. Our team excels at resolving complex scientific problems with a systematic approach and in a time-bound manner while assuring the quality of all processes and products.Syngene’s development capabilities include Regulatory starting materials (RSMs), APIs, High Potency APIs, NCEs, Novel advanced intermediates and oligonucleotides with therapeutic and diagnostic applications, from laboratory to manufacturing scale of 100 g to 500 kg. Our expertise also includes working with performance chemicals and specialty materials using synthetic organic chemistry and polymer chemistry. -
Brochure Syngene -Biologics Development
Syngene is an integrated research, development, and manufacturing services organization offering biologics solutions for human and animal health As a fully integrated custom biomanufacturer, our solutions include mammalian and microbial capabilities for clinical and commercial supply of drug substance and drug products
We have a strong track record in terms of both experience and know-how across mAbs, bispecific, ADC, antibody fragments, recombinant proteins, glycoproteins, mRNA, pDNA, microbial (E. coli and Pichia) and microbiome Live Biotherapeutic Product (LBP).
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance




















-file141696.jpg)















































.jpg)