Luer Vial by GEMÜ - Drug delivery device
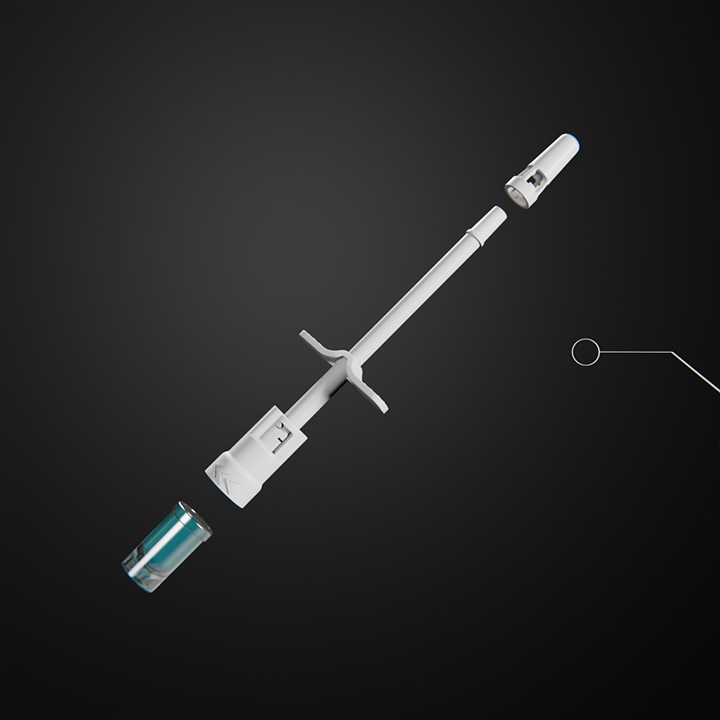
Luer Vial by GEMÜ
The new product from GEMÜ Medical. Easy application of medicine via the Luer Vial. For nasal, oral, topical and veterinary applications with customised spray heads.
Produced in a clean room to the highest standards and regulations.
GEMÜ presents the new Luer Vial. The use of the packaging and application system is simply flexible. The Luer Vial System impresses with its simplicity of use. Because sometimes less is more. The application attachments can be selected flexibly and adapted to customer requirements. In addition to the versatility of the application attachments, the vial offers the option of variable filling volumes between 0.2 and 0.6 milliliters.
The vial made of COC material has excellent barrier properties that are comparable to glass. The advantage is that the high-performance plastic is virtually unbreakable. The Luer vial can be used for a wide range of applications. Individual spray attachments allow liquid or semi-solid medication to be administered nasally, orally or even topically. The device can also be used without the spray head. The Luer connection makes it possible, for example, to combine the Luer Vial with a variety of standard components in infusion therapy.
We would like to invite you on a journey into the development of the Luer Vial. In our development department, our employees are working on an optimal solution for the Luer Vial. Nothing should be left to chance. Material selection, design and function of the application system were designed to meet the high demands of the primary packaging market. All development and design stages are accompanied by simulations and flow studies in order to identify potential risks at an early stage.
Be it for the system itself or its production. The result: tests deliver the desired results right from the start. Functional and extraction tests were successfully passed. The components of the Luer vial are manufactured using an injection molding process under ISO class eight clean room conditions. The individual parts are then assembled and the finished device packaged under the same conditions. The final packaging takes place in the logistics center.
The finished assemblies are then sent to our customers all over the world.

Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance