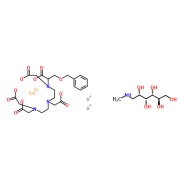
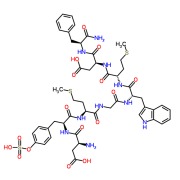
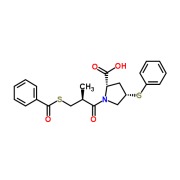
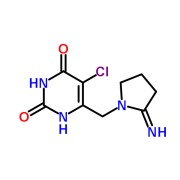
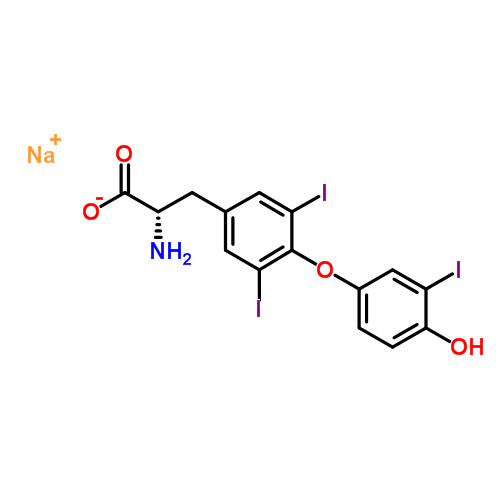
Liothyronine Sodium
Product Description
Biophore Pharma INC.

-
US
-
2015On CPHI since
-
3Certificates
Company types
Specifications
Biophore Pharma INC.

-
US
-
2015On CPHI since
-
3Certificates
Company types
More Products from Biophore Pharma INC. (57)
-
Product Temozolamide
Temazolamide is used in the treatment of Anticancer.
Biophore's Temozolamide: Polymorphic form; Form III, Stringent control on genotoxic impurities, Product meets USP specificationsm, All impurities below 0.15, DMF has been reviewed and approved in Europe, Particle size requirement as per c... -
Product Zileuton
Zileuton is an NSAID, and Anti Asthama agent.
Biophore's Zileuton: USFDA approved manufacturing facility, DMF Number 26446, Completeness Assessment of the US DMF Complete. -
Product Diazoxide
Diazoxide is an Anti hypoglycemic agent.
-
Product Eliglustat
Eliglustat is an Endocrine-Metabolic Agent is indicated for the Long-term treatment of adult patients with Gaucher disease type 1.
Biophore's Eliglustat: Under development
-
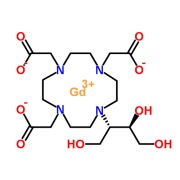
Product Gadobenate dimeglumine
Gadobenate dimeglumine is an Diagnostic Agent which is indicated for the treatment of Magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults.
Biophore's Gadobenate dimeglumine: API Developed, Stringent control on inorganic impurities, ... -
Product Gadobutrol
Gadobutrol is an MRI Contrast Agent.
Biophore's Gadobutrol: Product meets the specifications of the EP draft monograph, All impurities below 0.05, Stringent control on sodium and chloride. -
Product Methazolamide
Methazolamide Anti Coagulant indicated for the treatment of ocular conditions.
Biophore's Methazolamide: -
Product Nadolol
Nadolol is an Antihypertensive agent indicated for the treatment of long-term management of patients with angina pectoris.
Biophore's Nadolol: Lab development complete. Tech pack available. Validations under progress. DMF by Q3, 2016. -
Product Palbociclib
Palbociclib is an Cyclin-Dependent Kinase Inhibitor indicated for the treatment of hormone receptor (HR)-positive, human epidermal growth factor receptor 2.
-
Product Sincalide
Sincalide is an Diagnostic Agent indicated To stimulate gallbladder, pancreatic secretion, to accelerate the transit of a barium meal through the small bowel.
Biophore's Sincalide: API Developed, Validations Planned in Q3 2016, DMF to be filed in Q1 2017. -
Product Zofenopril
Zofenopril is an Antihypertensive agent.
Biophore's Zofenopril: USFDA approved manufacturing facility, US DMF Filed [ # 026446 ]. -
Product Tipiracil
Tipiracil is an Thymidine phosphorylase inhibitor/nucleoside metabolic inhibitor used to treatment of metastatic colorectal cancer.
Biophore Pharma INC. resources (2)
-
Video Biophore India Pharmaceuticals Pvt Ltd. at CPHI BCN 2023
Biophore India Pharmaceuticals Pvt Ltd. at CPHI BCN 2023 -
Video BioPhore India Pharmaceuticals Pvt Ltd at CPHI Worldwide 2018
Dr. Jagadeesh Babu Ranglsetty, Founder & CEO of BioPhore India Pharmaceuticals Pvt. Ltd., speaks With CPHI TV at CPHI Worldwide in Madrid, Spain
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance