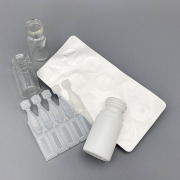
Inhaler Device Testing Services
Product Description
Oxford Lasers Ltd

-
GB
-
2021On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
Categories
Specifications
Oxford Lasers Ltd

-
GB
-
2021On CPHI since
-
1Certificates
-
50 - 99Employees
Company types
Primary activities
More Products from Oxford Lasers Ltd (2)
-
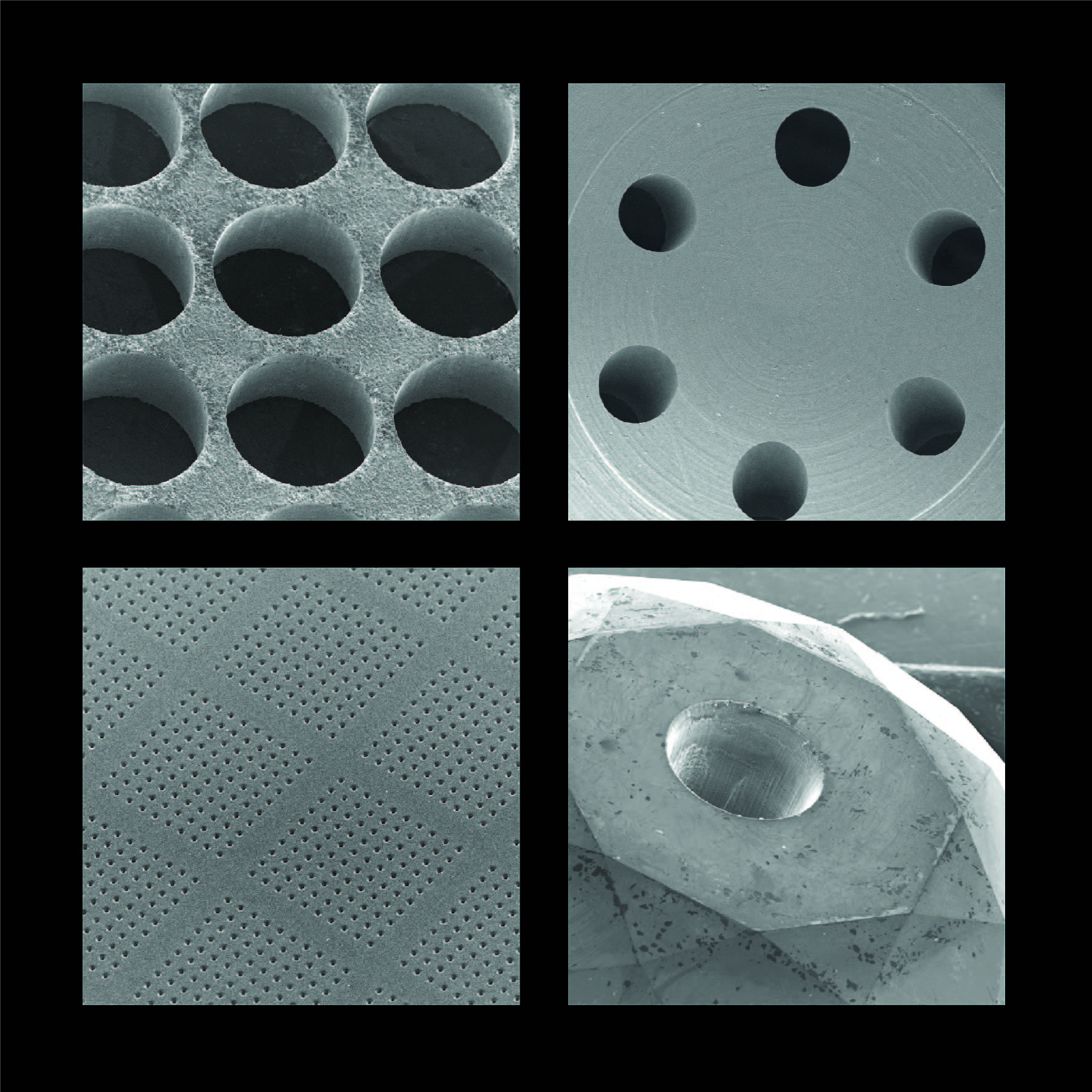
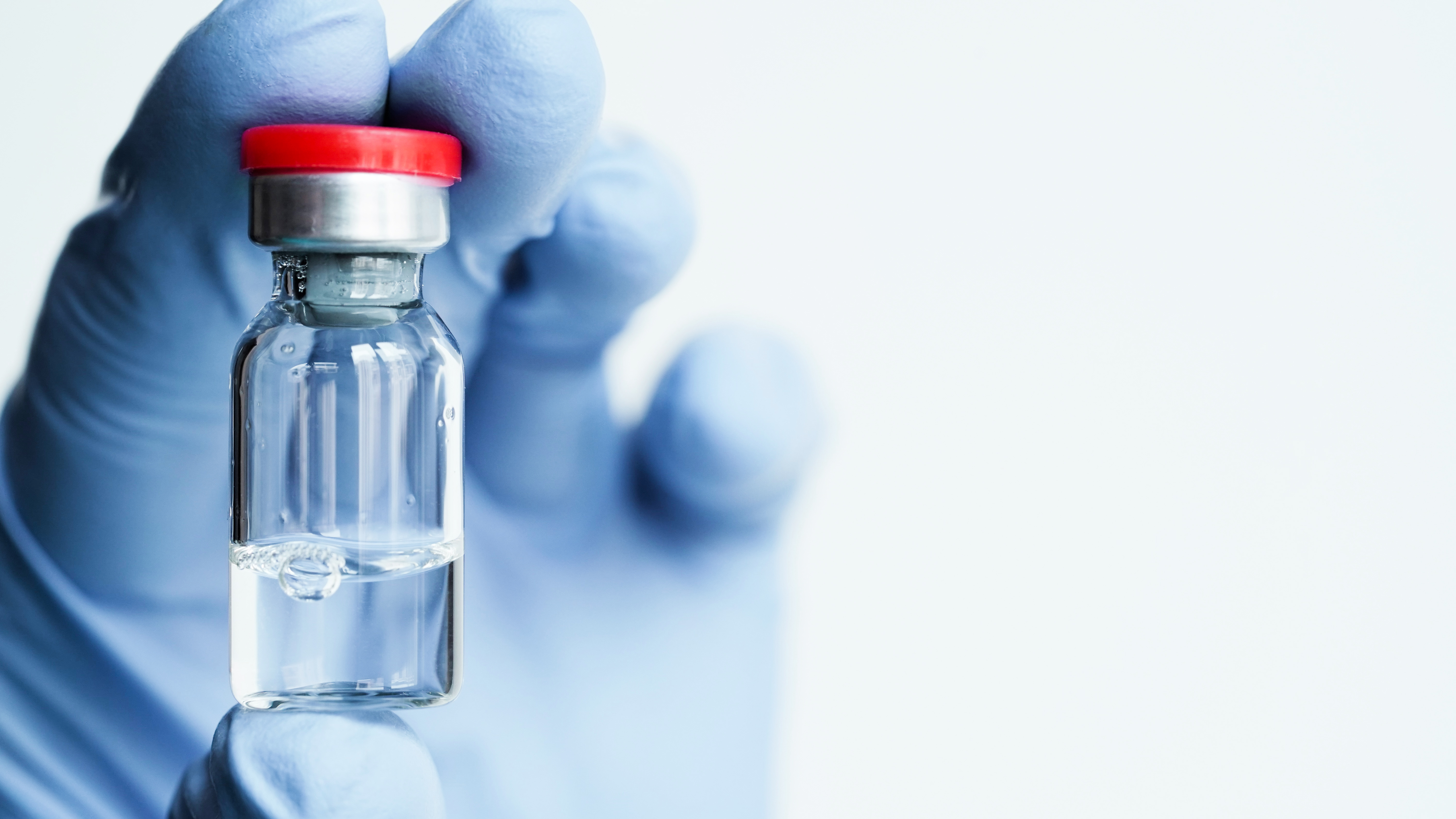
Product Laser Drilling Positive Controls in CCIT
Oxford Lasers is a world-class supplier of precision laser-drilling to create positive controls in container closure integrity testing (CCIT). Positive controls are created by drilling a known, measured micro hole in a package. These are used in the production process to provide deterministic proof that ... -
Product Laser Micromachining Services & Systems
From pinholes in stainless steel discs to complex arrays in semiconductor guide plates, we provide a wide range of ultra-high precision laser drilling, cutting, milling, scribing and ablation contract services (and systems) for numerous applications in industry & academia. With over 45 years of experie...
Oxford Lasers Ltd resources (3)
-
Brochure Catalogue - Laser Drilling Positive Controls CCIT
Oxford Lasers' full catalogue of capabilities and types of materials we laser drill in pharmaceutical packaging to create reliable positive controls for container closure integrity testing (CCIT). -
Brochure Precision & Consistency in Laser-Drilled Positive Controls CCIT
Handout describing Oxford Lasers' methodology of laser drilling CCIT positive controls to create precise and consistent calibrated micro holes down to 2 microns. Our micromachining techniques and knowledge provides confidence that our positive control packages produce consistent testing results across products, instruments, and sites with a robust audit trail making your manufacturing processes align with EU Annex 1, USP 1207 leak testing guidelines and FDA CGMP regulations. -
Brochure Inhaler Device Testing - Dynamic Performance Evaluation
Oxford Lasers' Imaging Division brochure describing our contact laboratory services in imaging and evaluation of inhaled drug delivery devices.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance

































































.jpg)









