CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
In vivo Pharmacokinetics of Biologics

Product Description
Pharmacokinetics and quantification research services:- Protein/mAb/ADC isolation and enzymatic digestion - UPLC/HR-MS or UPLC/MS/MS analysis of surrogate peptides - Mouse and rat pharmacokinetics - ELISA quantification
Admescope

-
FI
-
2018On CPHI since
Categories
Specifications
- Supplied fromFinland
Admescope

-
FI
-
2018On CPHI since
More Products from Admescope (1)
-
Product Structural Characterisation of Biologics
Structural characterization of proteins, monoclonal anti-bodies (mAbs) and antibody-drug conjugates (ADCs):- HR-MS based molecular weight measurement of intact proteins - Reduced / non-reduced forms (heavy and light chains of mAbs) - Peptide mapping
Recommended Products
-
Product R&D service
Neotron Pharma is able to offer you a research and development service. The research and development laboratory, operating under the ISO regime, will be able to develop customized methods for the customer in faster times and with lower costs. Subsequently, the customer will be able to validate the GMP meth...
-
Product Bioanalytical Services (GLP/GCP)
Services for Bioanalysis (GLP/GCP): In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported...
-
Product Large molecule bioanalytical services
Intertek offers wide range of pharmaceutical services which includes large molecule bioanalytical services. It includes immunochemistry services, quantitative immunoassays, immunogenicity assays, biomarkers assays and validation, cell-based neutralization assays, ligand-binding assays, etc.
-
Product Clinical Trials and Bioequivalence Studies, Clinical Services, Bioanalysis
ACDIMA BioCenter is a comprehensive clinical CRO based in Jordan and founded in 2000. ACDIMA BioCenter is a multinational investment operates as business-driven model with focus on supporting the industry of clinical trials and bioequivalence studies services that help expediting their regulatory a...
-
Product Excipia Experts in excipient analysis, characterization and deform...
Excipients can have an important impact on the manufacturability and pharmaceutical performance of a formulation. It’s therefore important to identify and control functionality related characteristics (FRCs) of excipient materials in order to achieve safe, robust and stable products. Expe...
-
Product Sucralfate tablets
sucralfate, sold under various brand names, is a medication used to treat stomach ulcers, gastroesophageal reflux disease, radiation proctitis, and stomach inflammation and to prevent stress ulcers. Its usefulness in people infected by H. pylori is limited.
-
Product Clinical Solutions: Bioanalysis of small and large molecules
We provide a wide range of GCP/GLP bioanalytical services for preclinical and clinical studies with human and/or veterinary drug products up to Phase III. We offer development and validation of bioanalytical methods in different biological matrices; complete bioequivalence studies; pharmacokinetics/toxicok...
-
Product Regulatory Testing and Research based services for Pharmaceuticals, Chem...
1) Analytical Testing Of Pharmaceuticals & Cosmetics2) Biotechnological Services
3) Microbiological Services
4) Bio - Compatibility Studies of Medical Devices(As per ISO 10993USFDA and MHLW Guideline)
5) Preclinical & Toxicological Services
6) Phytochemical & AYUSH Testing Se...
-
Product Development Sciences
Our development laboratories are well equipped for conducting a diverse range of experiments. These encompass formulation development, cycle design and process refinement, as well as evaluating finished product. Ease of scale-up is accomplished by completing process development studies within a pilot scale...
-
Product Bioequivalence tests for topical drugs
We are a GLP lab specialist in vitro permeation testing, including the bioequivalence tests under the EMA draft guideline. We support pharmaceutical companies through bioequivalence studies. Visit our booth and find out more about our R&D dedicated to this topic.
-
Product Contract R&D and Scale-Up
Actylis offers GMP, biopharma and non-GMP specialty chemical custom ingredient research and development services by generating robust, practical and cost-effective processes for the preparation of specialty chemicals, excipients, and pharmaceutical ingredients. With decades of experienc...
-
Product Stability Studies
Solvias offers the complete range of stability studies according to ICH guidelines for drug development and follow-up stability studies, covering all ICH standard storage conditions as well as low temperature storage. All our processes take place on one connected site, where there is a high degree of QC ex...
-
Product Colony Counter and Documentation System with 21 CFR Part 11 Compliance
Colony Counter and Documentation System with 21 CFR Part II Compliance Software • Automated colony count in seconds. • Documentation of Colony for future Reference. • Minimum operator interaction • Meets FDA 21 CFR Part 11 Compliance • Compact system utilizes incident, tra...
-
Product Bioanalysis of Small Molecules
Kymos Pharma Services, S.L. offers wide range of bionalysis services in medicinal chemistry which includes bioanalysis of small molecules. Kymos offer bioanalytical method development and validation of innovative small molecules by mass spectrometry. It has the experience of being the selected partner for ...
-
Product Structural Characterisation of Biologics
Structural characterization of proteins, monoclonal anti-bodies (mAbs) and antibody-drug conjugates (ADCs):- HR-MS based molecular weight measurement of intact proteins - Reduced / non-reduced forms (heavy and light chains of mAbs) - Peptide mapping
-
Product Quality Control and Release Analysis
Solvias provides wide range of analytical services which includes quality control and release analysis. The analyses are routinely performed at Solvias according to GMP quality standards for drug substances & products, intermediates, starting materials and excipients. Contact us for more information.
-
Product Biocompatibility of Medical Devices according to ISO 10993-1 series
Galileo Research can drive the nonclinical development of your medical device by preparing the Literature Report and the Biological Evaluation Plan (BEP) according to ISO 10993-1, performing additional tests, if needed, and releasing the Biological Evaluation Report (BER).
All tests can be condu...
-
Product Absorption studies of Dietary Suplements and Phytopharmaceuticals
Kymos Pharma Services, S.L. offers wide range of bionalysis services in medicinal chemistry which includes absorption studies of dietary suplements and phytopharmaceuticals. It performs the bioanalysis of active ingredients in biological samples coming from clinical studies of nutraceuticals, medical devic...
-
Product Pharmaceutical Reference Standards
All Pharmaceutical Reference Standards are supplied with Certified COA along with supporting analytical data like HPLC, Mass, NMR, CMR, UV, IR, TGA, ROI, CHNS.
-
Product Solid state screenings & investigations
• These studies bring together knowledge on the nature of the solid states of a given compound (intermediates or APIs) and its associated properties (stability, solubility, dissolution) in order to select the right solid phase. • Screening operated: salts, cocrystals, polymorphs and amorphous solid dispe...
-
Product Oligonucleotide Synthesis Service and Partnerships
The use of oligonucleotides is rising for the diagnosis and treatment of illnesses. Cutting edge research often requires complex oligonucleotides, the synthesis of which requires a deep understanding of nucleic acid chemistry. Our experienced scientists can synthesize complex oligonucleotides for your imme...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materia...
-
Product GCP/GLP Bioanalysis Services
In our state-of-the-art facilities, we provide GLP/GCP method development, validation, method transfer, sample analysis and pharmacokinetic and toxicokinetic support, along with automated data collection and reporting systems. Over the past 20 years, we have supported the development of pharmaceuticals, bi...
-
Product Drug safety, in vitro and In vivo studies
In support of human clinical trials and marketing authorization approval, Galileo Research offers a full battery of toxicity studies for the regulatory development of small drugs, as well as biologics and advanced therapies, in compliance with Good Laboratory Practice (GLP), ICH and OECD guidelines.

-
Product Contract Research Organization (CRO)
PI Health Sciences is your strategic partner in cutting-edge CRO Services. We specialize in core chemical discoveries, synthesis, analytical research, and more. Trusted by global pharmaceutical giants, we prioritize quality, IP protection, and data security. Our expertise spans from drug discovery to comme...
-
Product Biotherapeutics
To accelerate the development and marketing of your Biotherapeutics, Quality Assistance offers a complete analytical package to meet the EMA and FDA requirements, all on one site.
Whether it is to extend your analytical capacities or to outsource parts or all of your analytical needs, ...
-
Product Evotec INDiGO ®
Accelerated drug development through interdisciplinary integration and expert coordination of all drug development activities “under one roof”
Industry-leading timeline from candidate nomination to regulatory submission
Custom-designed, flexible development plans allow for rea...
-
Product Custom Synthesis
Some of the key services we offer include:
# Custom Synthesis
# Sustainable Process Development
# Medicinal Chemistry
# CRAMS (Contract Research and Manufacturing Services)
# R&D Digitization: Implementation of LN and LIMS
-
Product Oligonucleotide Synthesis Service and Partnerships
The use of oligonucleotides is rising for the diagnosis and treatment of illnesses. Cutting edge research often requires complex oligonucleotides, the synthesis of which requires a deep understanding of nucleic acid chemistry. Our experienced scientists can synthesize complex oligonucleotides for your imme...
-
Product Integrated Novel Drug Design and Development
- From concept to market- Computer aided design, QSAR & molecular modeling
- Lead structure - Biological testing - Drug development
- Pre-clinical and Clinical Development
-
Product Pharmacokinetic modeling
The SurroGUT™ platform is a powerful tool for developing advanced predictive Physiologically Based Biopharmaceutics (PBB) models and mechanistic in vitro–in vivo correlations (IVIVCs). It uniquely combines TIM dissolution experiments, TIM gastrointestinal fluids, cell-based microfluidics, and GastroPlus® s...
-
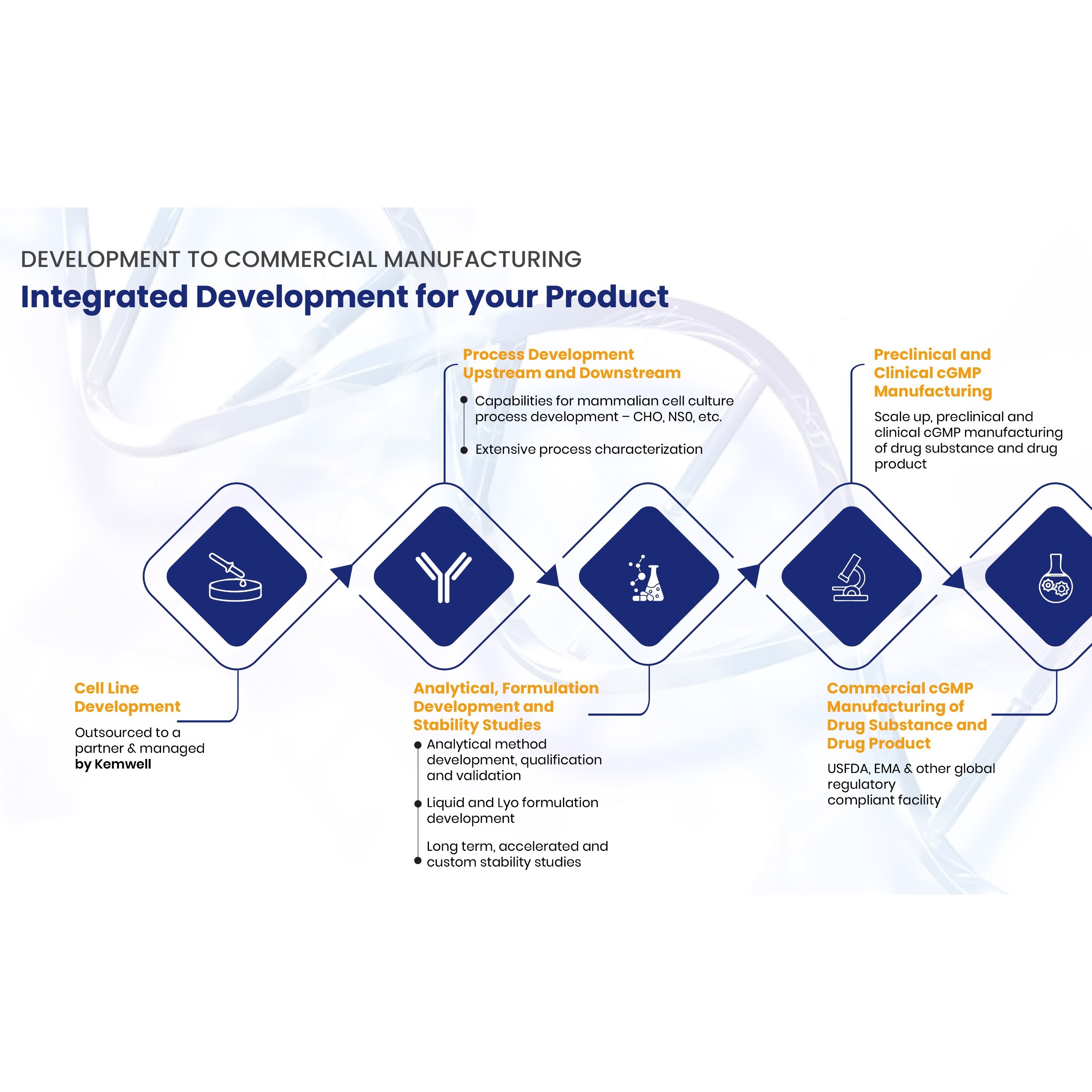
Product Kemwell Biopharma
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufa...
-
Product ADAPTEK® TECHNOLOGY
ADAPTEK® TECHNOLOGY is the general name for our 4 international patents and expertise in nanotechnology and controlled release systems.This is a transversal technology that allows to develop customized biopolymeric nanohydrogels, allowing the load with different API (drugs, vitamins, growth factors, etc.)....
-
Product Microbiological Analysis
Oasis Test House caters to the microbiological requirements for its clients serving them with the below analysis. • Preservative Efficacy Analysis • Sterility Test • Sterility Validation • Microbial Limit Test Validation • Microbial count and pathogens • Bioburden testing • Antibiotics assay • Vitam...
-
Product List of Bioanalytical Methods
ACDIMA BioCenter is a comprehensive clinical CRO based in Jordan and founded in 2000. ACDIMA BioCenter is a multinational investment operates as business-driven mode with focus on supporting the industry of clinical trials and bioequivalence studies services that help expediting their regulatory approv...
-
Product Cell Based Assays
Kymos Pharma Services, S.L. offers wide range of bionalysis services in medicinal chemistry which includes cell based assays. Expertise in cell biology enables us to implement, validate and perform cell-based assays (cba) for several applications like potency assays or neutralizing antibodies. Kymos work w...
-
Product CMAC - Secondary Processing Services
Formulation platforms investigating solid oral dosage forms - extrusion, blending, compaction and milling
CMAC National Facility provides: - Dedicated technical support within a World Class Manufacturing Research Facility - Unique expertise in advanced crystallisation, process development, f...
-
Product Bioanalytical Services
To effectively support your drug development project and maximize your R&D productivity, NUVISAN offers a wide array of bioanalytical solutions for small and large molecules. Starting at the discovery, all the way through phase IV, we offer expertise bioassays and high-sensitivity ligand-binding assays...
-
Product Analytical Services & Methods
CritiTech provides a suite of material characterization and analytical services to support our clients drug development projects from proof-of-concept through cGMP production. Our testing data and documentation is reviewed by experienced technical and quality staff to ensure it can be used to support regul...
-
Product Bioanalytical Services
Scorpius BioManufacturing develops custom assays to profile your molecule and test your clinical trial samples, allowing the seamless progression of your product throughout its lifecycle. Beginning with the end in mind, we design pre-clinical analytical test methods and IND-enabling programs that easily ...
-
Product Analytical Services
Solvias provides cGMP-compliant contract analytical services to help you ultimately provide safer products to consumers. We provide a comprehensive range of analytical services to the pharmaceutical, biotech, medical devices and cosmetics industry with similar regulatory requirements for raw materials, int...
-
Product Inhalation Product Analysis and Testing
Inhalation product analysis and testing services include particle size and droplet characterization, delivered dose testing, as well as aerodynamic particle size analysis – for pressurized metered-dose inhalers (PMDI), dry powder inhalers (DPI), nebulizers, and soft mist inhalers. Our Good Manufacturin...
-
Product Method Development
We are specialist in the method development of analytical methods for analysis of API, Final Drug Products or low level impurities. Usually followed by full validation in accordance to ICH guidelines.
-
Product Comparability Studies for Biopharmaceuticals
We design bespoke analytical programmes that ensure that the relevant quality attributes for your drug substance or product are evaluated to support your manufacturing process changes. We select analytics referenced by the ICH Q6B to examine the product's structural features (primary, secondary and&nb...
-
Product Cell-based Neutralization Assays
Experienced cell-based neutralizing antibody assay capabilities. Our experts, at our GLP/GCP compliant laboratory, evaluate each therapeutic, conduct tailored assay development and ensure that each assay is optimized to suitably determine the presence of NAb with maximum possible level of drug toleran...
-
Product Analytical Services (GMP and R&D)
Our analytical portfolio comprises 200+ different techniques – all performed by Coriolis’ experts in-house. Our offering includes:
• Aggregate analytics • Particle characterization • Particle identification • Surfactant characterization • Higher order structure analysis • Funcional assays • Chemi...
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance