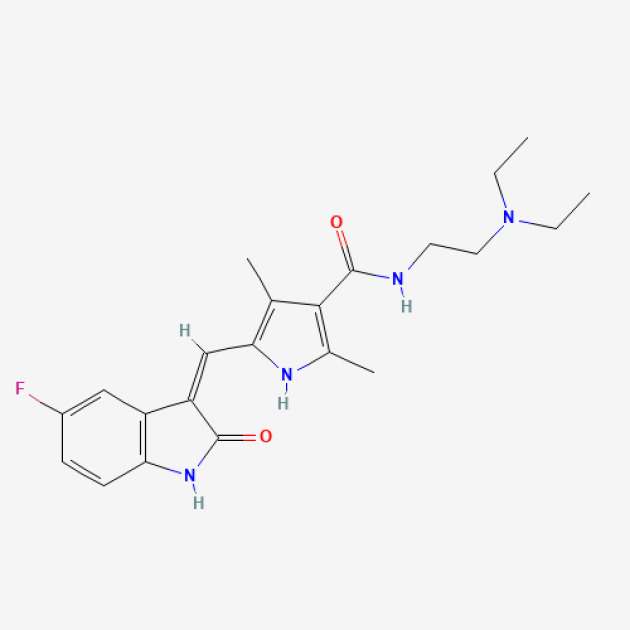
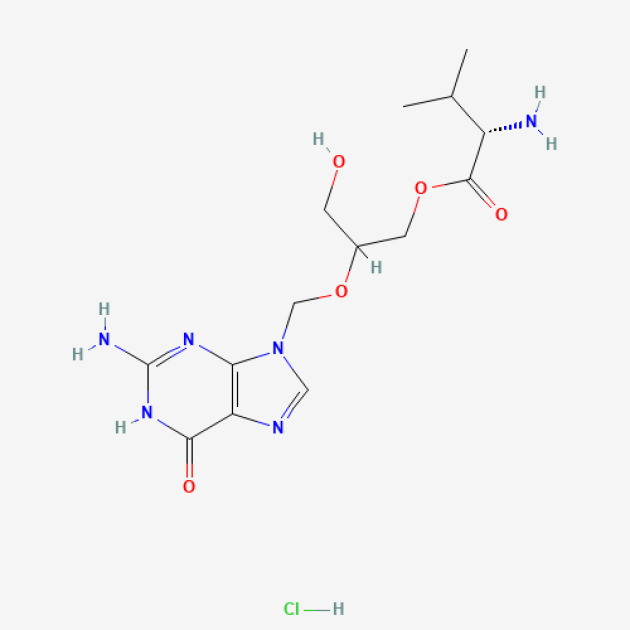
Imatinib Mesylate-API

Product Description
EXAMON Handelsges.m.b.H.

-
AT
-
2024On CPHI since
Categories
Specifications
EXAMON Handelsges.m.b.H.

-
AT
-
2024On CPHI since
More Products from EXAMON Handelsges.m.b.H. (32)
-
Product Dasatinib anhydrous/monohydrate-API
• High-purity active pharmaceutical ingredient designed for the manufacture of dasatinib-based formulations. • A potent multi-targeted tyrosine kinase inhibitor effective against BCR-ABL and Src family kinases. • Exhibits robust inhibitory activity, making it a critical component in treatments for CML a... -
Product Desloratadine-API
• High-quality active pharmaceutical ingredient used in the manufacture of non-sedating antihistamine formulations. • Manufactured under strict cGMP standards to ensure exceptional consistency and purity. • Offered as a fine, crystalline powder with excellent physicochemical stability. • Complies with ... -
Product Enoxaparin sodium-API
• High-quality active pharmaceutical ingredient used in the production of low molecular weight heparin formulations. • Exhibits reliable anticoagulant activity with a favorable pharmacokinetic profile for clinical applications. • Manufactured under stringent cGMP conditions to guarantee exceptional puri... -
Product Erlotinib hydrochloride-API
• High-purity active pharmaceutical ingredient developed for erlotinib-based formulations. • Potent inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, essential for targeting aberrant cancer cell signaling. • Supplied as a fine crystalline powder with excellent physicochemical sta... -
Product Fingolimod hydrochloride-API
• High-quality active pharmaceutical ingredient developed for fingolimod-based formulations. • Potent modulator of sphingosine-1-phosphate receptors, crucial for managing relapsing multiple sclerosis. • Manufactured under strict cGMP conditions to ensure consistent quality and batch-to-batch reproducibi... -
Product Gefitinib-API
• High-quality active pharmaceutical ingredient developed for gefitinib-based formulations. • Selective EGFR tyrosine kinase inhibitor designed to block aberrant cancer cell signaling. • Offered as a fine, crystalline powder with excellent purity and consistent physicochemical properties. • Manufacture... -
Product Lenalidomide hemihydrate-API
• High-quality active pharmaceutical ingredient designed for lenalidomide hemihydrate formulations. • Manufactured under stringent cGMP conditions to ensure batch-to-batch reproducibility and high quality. • Extensively characterized using advanced analytical techniques such as HPLC, NMR, and LC-MS for ... -
Product Letrozole-API
• High-quality active pharmaceutical ingredient designed for letrozole-based formulations. • Provided as a highly purified, white crystalline powder with consistent physicochemical properties. • Manufactured under strict cGMP conditions to ensure reliable quality and batch-to-batch reproducibility. • E... -
Product Pazopanib hydrochloride-API
• High-purity active pharmaceutical ingredient engineered for pazopanib-based formulations. • Offered as a fine crystalline powder with robust purity and consistent physicochemical properties. • Produced under rigorous cGMP standards to ensure batch-to-batch reproducibility and quality assurance. • Sub... -
Product Sunitinib malate-API
• High-quality active pharmaceutical ingredient developed for sunitinib-based formulations. • Provided as a fine crystalline powder with exceptional purity and consistent physicochemical attributes. • Manufactured under strict cGMP conditions to ensure uniform quality and batch-to-batch reproducibility.... -
Product Valganciclovir hydrochloride-API
• Premium-grade active pharmaceutical ingredient formulated for valganciclovir-based products • Supplied as a finely milled crystalline powder with exceptional purity and consistent quality • Produced under strict cGMP conditions to ensure reproducibility and high manufacturing standards • Extensively ... -
Product Erlotinib-KIMOTAR®
• Erlotinib blocks the epidermal growth factor receptor’s tyrosine kinase to suppress tumor cell growth. • Validated efficacy in non-small cell lung cancer (first-line). • Offered in tablet form to facilitate patient-friendly, outpatient treatment. • Underpinned by a wide range of peer-reviewed studies...
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance