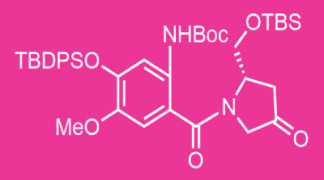
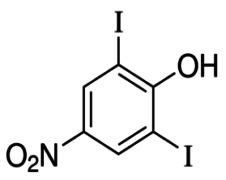
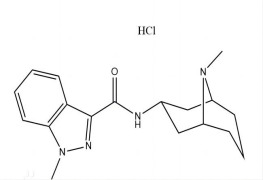
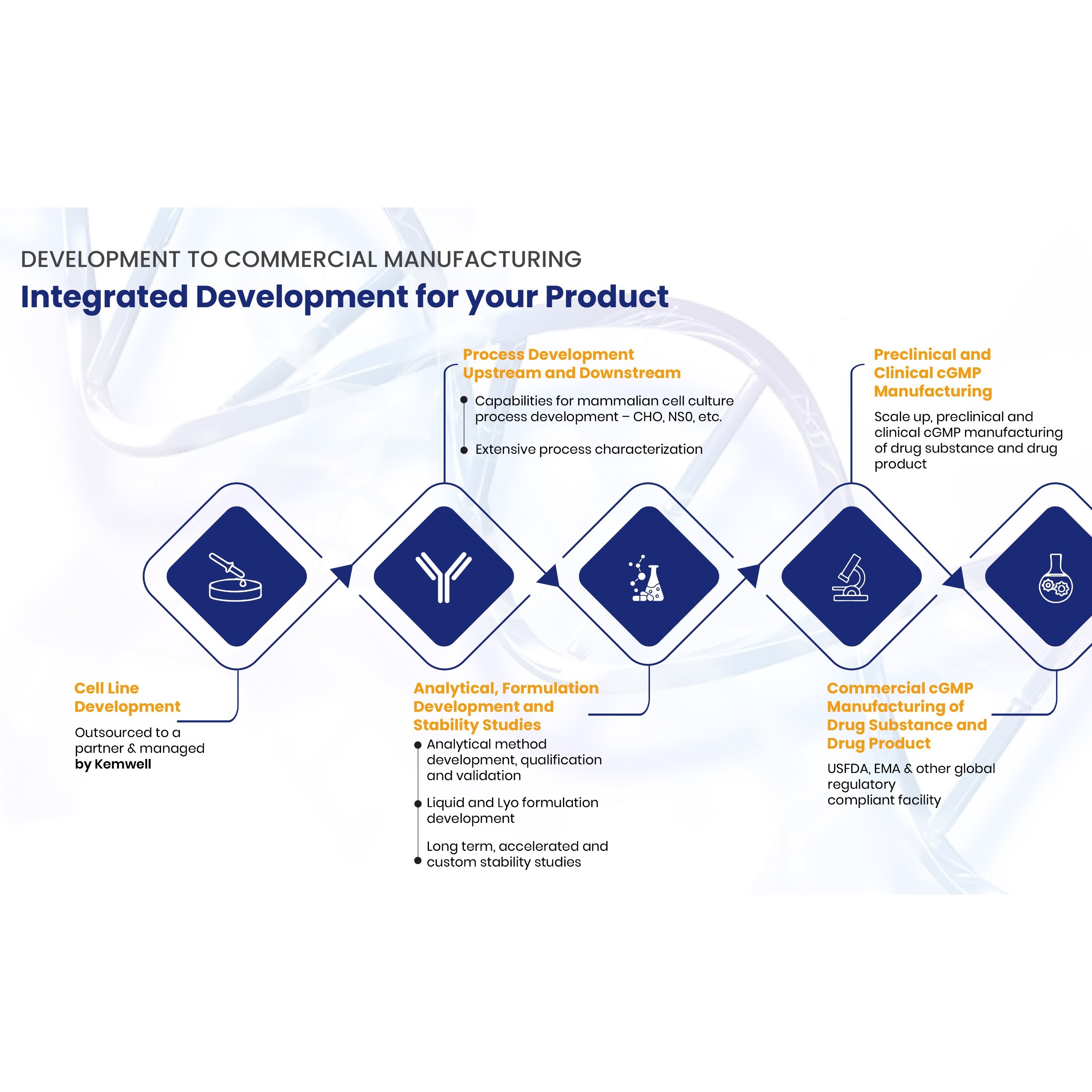
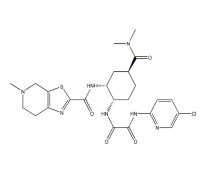
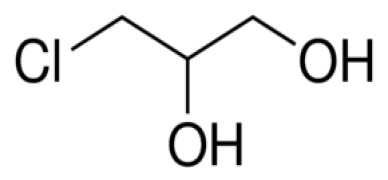
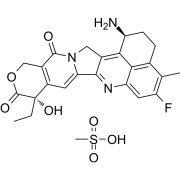
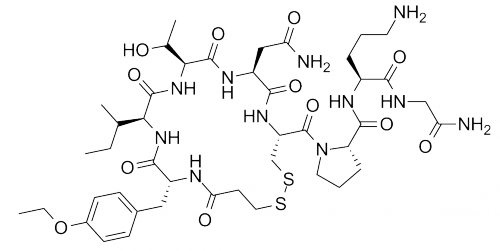Highly Potent API Handling

Product Description
Lonza Capsules & Health Ingredients
-
US
-
2015On CPHI since
-
250 - 499Employees
Company types
Primary activities
Categories
Lonza Capsules & Health Ingredients
-
US
-
2015On CPHI since
-
250 - 499Employees
Company types
Primary activities
More Products from Lonza Capsules & Health Ingredients (52)
-
Product TC 100 Insect Medium with L-glutamine
Lonza Biologics Inc offers wide range of pharma products and services which includes tc 100 insect medium with l-glutamine. It belongs to cell culture products, classical media, insect media services and products category. Contact us for more information. -
Product Unprocessed human bone marrow
Lonza Biologics Inc offers wide range of pharma products and services which includes unprocessed human bone marrow. It belongs to primary and adult stem cells adult stem cells & media services & products category. Contact us for more information. -
Product Validation package
Lonza Biologics Inc offers wide range of pharma products and services which includes validation package. It belongs to downstream products and services category. It comprehensive package represents multiple analysis conducted by Lonza Biologics Inc, along with the support of qualified vendors, to determine... -
Product Solid Form Services
The selection of a lead solid form of an API is a critical step in early pharmaceutical development. Determining the most suitable chemical and physical form impacts a range of critical parameters including manufacturability, stability and bioavailability. The selection of a lead solid involves the screeni... -
Product Winkqcl™ endotoxin detection & analysis software
Lonza Biologics Inc offers wide range of pharma products and services which includes winkqcl™ endotoxin detection & analysis software. It belongs to pharma & biotech informatics services and products category. Features: integration of molecular devices® spectramax®, gemini™ and versamax™ r... -
Product Active Pharmaceutical Ingredients and Intermediates
We provide customized chemical synthesis for active pharmaceutical ingredients from concept to commercial supply.
We are an industry-leading integrated development and manufacturing service provider for small molecule drug substances and their intermediates.
Chemistry is c... -
Product Bioavailability Enhancement - Oral Drug Products and Intermediates
The depth of our bioavailability (BA) enhancement offering makes us a leader in addressing addressing low solubility, low bioavailability and dissolution rate issues. We work collaboratively with our customers to advance compounds, or re-purpose existing compounds, across a full range of API properties and... -
Product Inhalation Formulation Services
Inhaled drug products for nasal or pulmonary delivery continue in popularity due to the prevalence of respiratory diseases around the globe. Additionally, the lung’s absorptive capacity continues to be explored as an attractive delivery point for both local and systemic applications. Particle engineering, ... -
Product Microreaction Technology
Use of Microreaction Technology (MRT) may lower product cost of goods and increases yield and throughput of a manufacturing facility. This technology is able to perform hazardous, high temperature and pressure reactions, as well as those containing unstable or extremely reactive elements. Using MRT, these ... -
Product Bioassay services
At any point along your product development path, Lonza—s Bioassay Services can help clients with immediate support for specific biological assay development. We work with our clients to overcome any quality, regulatory, or security challenges. For biopharmaceutical and cell therapy manufacturing, L... -
Product Biological assays
Lonza Biologics Inc offers wide range of pharma products and services which includes biological assays. It belongs to pharma & biotech, bioassay services category. It includes cell proliferation, cell permeability, apoptosis, cell differentiation, cell survival/cytotoxicity, immunoassays: elisa, flow cytom... -
Product Biopreservation, fill and finish
Lonza Biologics Inc offers wide range of pharma products and services which includes biopreservation, fill and finish. It belongs to cell therapy products and services category. It recognize the unique time constraints associated with converting a cultured cell harvest into a formulated cell therapy product.
Lonza Capsules & Health Ingredients resources (44)
-
News CPHI North America 2023 – From the Floor
Follow along for live updates from the Content team as we bring you the latest from CPHI North America 2023 - from session talks, panel discussions, interviews, and more, there's a lot to discover with CPHI Online at the Pennsylvania Convention Center! -
News Lonza signs five-year collaboration deal with VC firm Bioqube to speed up portfolio companies’ development and manufacturing
The offering will accelerate timelines for the development and manufacturing of molecules and disruptive technologies -
Video Lonza Quakertown - Micronization and Particle Size Control
Lonza Quakertown provides expertise in particle size reduction and classification servicing the cGMP pharmaceutical marketplace. The presentation will provide a description of the site and an overview of capabilities specific to micronization and classification while highlighting the value of particle size reduction and control. The basic mechanics of jet milling will be explored along with process development and process scale-up. The presentation will conclude with a discussion of the option of combining micronization services with other platforms and technologies within Lonza as an integrated service offer. This session was originally broadcast live as part of CPHI North America 2021 -
News Lonza Switzerland site to undergo expansion of microbial development capabilities
The expansion includes the installation of a pilot suite with a 50-L fermenter and automation upgrades to accelerate clinical and commercial projects -
Video Lonza Tampa - Expansions in HPAPI for Oral Solid and Inhalation Technology
Our facilities in Tampa (FL) US are an integral component of Lonza’s design, development and manufacturing network for drug substances and specialized drug products. Working collaboratively with our customers, our Tampa team designs develops and manufactures oral solid and inhalation drug products. The site provides a full range of services, starting from the initial stages of product development including pre-formulation, formulation & process design, and analytical method development. These services extend into early phase clinical, late phase clinical, and commercial-scale production including packaging and distribution worldwide. Tampa also serves as our US Center of Excellence for rapid first-in-human services utilizing Powder-in-Capsule (PIC) based on our proprietary Xcelodose® Precision Powder Micro-Dosing Technology to effectively increase speed-to-clinic initiatives. Site accomplishments: PIC to Commercial (ALUNBRIG), DEV to Commercial +$20M in renovation Site center of excellence: FIH Initiatives for Speed to Clinic Unit operations same for inhalation (XD, Harro) Cross site case studies (TBD) – VISP/QT/TPA Site expansion: Containment & Unit Operations OEB levels and containment (multi-use, unit op specific) Extension of service: Inhalation Testing Leverage Bend CoE for inhalation development Analytical capabilities employed 2021 This session was originally broadcast live as part of CPHI North America 2021 -
News Lonza boosts exosome capability with Codiak BioSciences facility acquisition
The companies will establish a Centre of Excellence focused on exosome manufacturing and characterization technologies -
Brochure Presentation: Achieving Bioavailability Enhancement for Poorly Soluble Compounds
A large and increasing fraction of orally administered small molecules in pharma company pipelines have poor oral absorption due to low aqueous solubility or dissolution rate. To address this trend, evaluating formulation approaches for increasing oral bioavailability are becoming routine early on in formulation development programs. Selecting the appropriate technology for your compound requires careful consideration of the compound physicochemical properties, target dose and pharmacokinetic profile, as well as overall market image. In this on-demand webinar, hear about some of the most common bioavailability-enhancing technologies for poorly soluble compounds and a strategic framework for identifying and selecting the appropriate technology. A case study is presented. -
News Lonza to establish multi-product fill and finish line in China
The new production line at Guangzhou site will supply global and domestic companies with clinical and commercial batches -
Whitepaper White Paper: SimpliFiH®️ Solutions for Accelerated Pharmaceutical Development
With the pharmaceutical industry’s focus on accelerating all aspects of drug discovery and development, it is crucial to reduce the time from initial product concept to first-in-human clinical completion. Numerous factors can slow this process, but challenges can be overcome by aligning with a single, integrated Contract Development and Manufacturing Organization (CDMO) to reduce the time, complexity, risks, and costs associated with engaging multiple partners. -
News Tech transfer: a many-layered artform that demands precision and communication
Tech transfer is a crucial phase that converts clinical promise into commercial gain but pressure on sponsors to reduce time to market in the age of COVID-19 means CDMOs have to be on top of their game to deliver. What are the latest innovations to solve tech transfer challenges? -
Brochure Presentation: Solid form screening and rational cocrystal design
Solid-form screening and characterization is a crucial part of drug substance development and pre-formulation. In this webcast, experts will discuss many aspects of solid-form screening including in-silico screening, which can save time and speed up the solid-form development process. A case study will review the in-silico screening of cocrystals of a pharmacophore using hydrogen bond energies and hydrogen bond propensities. A second case study will examine the cocrystallization of nutraceuticals and their versatile use as conformers. -
News CPHI Trend Report - CDMO Opportunities in the Chinese Market
Opportunities for contract development and manufacturing organisations (CDMOs) in China continue to grow but many questions remain over what the future of the sector will look like in the world's second largest pharmaceutical market. -
Webinar From Powder to Particles with Jet Milling
Jet milling is a well-established particle engineering technique for producing micronized powders with controlled particle size distribution and increased surface area. This makes them suitable for inhalation drug delivery. However, successful implementation of jet milling requires a robust Quality by Design (QbD) approach that ensures the critical quality attributes of the micronized particles are maintained throughout the process development. This presentation will cover the principles and intricacies of the jet milling process. It will also highlight some of the challenges when micronizing for inhalation delivery, and give some possible solutions. A case study will demonstrate a reliable process development strategy using a QbD approach for micronizing nilotinib, an API with potential for the treatment of severe chronic asthma. It will highlight the significance of implementing a QbD approach, and its benefits in process development. Overall, this presentation aims to provide valuable insights into the jet milling process and QbD approach for inhalation drug delivery and their potential applications in drug development. This presentation aims to provide an in-depth understanding of the jet milling process, and the significance of implementing a Quality by Design approach. It will address the intricacies of developing micronization processes as a particle engineering technology for inhalation. A case study will be presented to illustrate a reliable process development strategy that ensures all quality-critical attributes are maintained. -
News Discover the CPHI North America Learning Labs: Part One
Explore the series of Learning Labs at CPHI North America across several product innovation categories in which thought leaders at our exhibitors showcase their extensive expertise in all areas of the pharma supply chain, offering industry insights across drug manufacturing, outsourcing, pharma ingredients, drug delivery and packaging. -
Webinar "Panel: Looking to the Future – What’s Next for the Generic Drugs Market?"
Hear from our expert panel on the latest developments within the generic drugs market: Market outlook – what’s next for generics? Tackling shortages with fresh formulation Driving quality improvements through process control optimisation -
News Lonza to invest in mid-scale API manufacturing expansion at Chinese facility
The expansion of mid-scale capacity will enable the company to offer a smooth transition between early-phase and large-scale commercial production -
Webinar The Future of EU Off-Patent API Manufacturing
The European region has historically maintained a prominent role in global off-patent API manufacturing. However, it is apparent that unique constraints are linked to API manufacturing in the European market. In this presentation, we will delve into:
Exploring the challenges and opportunities of off-patent API manufacturing in Europe The significance of fortifying local production capabilities to bolster the European competitive edge in this sector Providing valuable insights that contribute to shaping the future of off-patent API manufacturing in Europe, ensuring resilience at a local level while maintaining global relevance -
News Pandemic creates extra demand for CDMO services but investment decisions remain key to success
While the COVID-19 pandemic has created even greater demand for partnerships, CDMOs need to be aware that the needle has shifted, creating both opportunities and challenges and changing how the services industry does business -
Webinar Keynote Address: Formulating the Future
Join us, where Ferenc Marofka rom the European Commission will provide an overview of the Drug Formulation market in Europe -
News Lonza to install new production line at Geleen to increase Moderna COVID-19 vaccine output by 300 million doses per year
Drug substance production line expected to be operational by end of 2021 as part of CDMO’s expanded collaboration with biotech
-
Webinar Strategies for Formulating Future Drug Modalities
Many promising drug candidates face abandonment in the development pipeline, often due to difficulties encountered during formulation phase or API delivery. Overcoming these challenges is crucial for successful drug development and commercialization. In this talk, Camille Dumont, Manager, Customer Applications at Lonza Capsules & Health Ingredients will highlight:
Actionable insights on how to overcome challenges around your future drug developments including your formulation and API delivery The advantages offered by Capsugel® Enprotect® capsules and Launch with Lonza™ Application Lab Services How Lonza can help with your next development project -
News New Moderna agreement will double vaccine drug substance production at Visp, says Lonza
Three additional drug substance manufacturing lines will be added to existing ones at Swiss site and are expected to be operational in early 2022 -
Webinar Leverage the Benefit of Drug Delivery and Formulation to Achieve Acceptability in the Target Patient Population
Acceptability of the pharmaceutical product is an essential part of patient centric drug delivery systems and formulation. Join to understand the importance of involving patients to achieve real “innovation” in drug product design and how I can be implemented in future drug product development.
Acceptability in the context of regulatory science The importance of patient involvement in drug product development and therapeutic outcomes -
News Lonza to build small molecule manufacturing complex at Visp site
CHF 200 million investment will include dedicated line for antibody-drug conjugate payload molecules and is scheduled to start operations in Q3 2023 -
Webinar Implications of FDA’s Quality Maturity Model (QMM) Initiative for API Manufacturers, Drug Sponsors and Drug Manufacturers
FDA’s Center for Drug Evaluation and Research’s (CDER)’s Office of Pharmaceutical Quality (OPQ), in collaboration with other stakeholders, is currently engaged in operationalizing a voluntary QMM rating program for domestic and foreign pharmaceutical manufacturers (2). Since first formally proposed in 2020, the QMM initiative has steadily gained momentum. This session will discuss:
The basic tenets of this new program Responsibilities for drug sponsors, CDMOs and API manufacturers as a result of the new program CDER’s lessons learned from their pilot program What to expect as this program continues to roll out -
News Lonza and Junshi Biosciences expand biologics manufacturing collaboration
The antibody-based products will be expressed using Lonza's GS Xceed Gene Expression System -
Video The Future of EU Off-Patent API Manufacturing
The European region has historically maintained a prominent role in global off-patent API manufacturing. However, it is apparent that unique constraints are linked to API manufacturing in the European market. In this presentation, we will delve into:
Exploring the challenges and opportunities of off-patent API manufacturing in Europe The significance of fortifying local production capabilities to bolster the European competitive edge in this sector Providing valuable insights that contribute to shaping the future of off-patent API manufacturing in Europe, ensuring resilience at a local level while maintaining global relevance -
News NextPharma completes purchase of Lonza lipid oral dosage form manufacturing sites
Ploermel and Edinburgh facilities employ around 390 permanent staff and will allow UK CDMO to expand its technology offering -
Video Making a Splash in the US Market: How PBPK Modeling is Playing a Central Role in Risk Reduction in Early Drug Development
Rapid and efficient development of drug candidates is increasingly important for pharmaceutical companies with accelerated timelines and funding constraints. However, many early drug candidates have poor oral absorption properties making it challenging to achieve target pharmacokinetic (PK) profiles. Without upfront knowledge of absorption risks and mitigation strategies, poor absorption can significantly impact preclinical and clinical study timelines and costs. Physiologically-based pharmacokinetic (PBPK) modeling software, such as the GastroPlus® platform from Simulations Plus, simulates dynamic physiological factors impacting oral performance. When coupled with in vitro measurements, PBPK modeling is effective in early development for 1) identifying absorption risks, 2) assessing the potential for solubility enhancing formulations such as salts, cocrystals, or amorphous solid dispersions to mitigate these risks, and 3) designing and optimizing preclinical and clinical studies with respect to dose, prandial state, or gastric pH modification to maximize the likelihood of achieving desired PK profiles. In this presentation, we will demonstrate how PBPK models combined with Lonza’s custom and off-the shelf in vitro tools and solubility enhancement expertise can be used to identify and mitigate absorption risks in early drug development, reducing the need for drug product reformulation or repeated preclinical or clinical studies. Key Learning Objectives: Learn how PBPK modeling can identify potential oral absorption risks and mitigation strategies (e.g. bioavailability enhancement) for early drug candidates. Learn how PBPK modeling coupled with in vitro testing can guide early selection of drug form and formulation to achieve clinical study goals. Gain insights into how key drug and formulation factors including solubility, permeability, and dissolution rate can impact absorption risks such as poor oral bioavailability, food-drug interactions, and pH-dependent DDI effects. -
News Lonza to support Pionyr Immunotherapeutics' oncology drug development
The CDMO will provide cell line development, process development, drug substance and drug product manufacturing for Pionyr’s monoclonal antibody candidate -
News Lonza to develop and manufacture Ixogen's oncolytic virus
PsiVac has generated compelling efficacy data in killing a broad range of tumour cells, including head and neck, bladder, liver, pancreatic and ovarian -
News Lonza boosts solid form services for small molecule APIs
The expanded and refined first-in-human services have been designed to meet the early-stage molecule development of small biopharma players -
News Lonza repositions as pure play healthcare firm after specialty unit sale
Private equity firms Bain Capital and Cinven to buy the CDMO's specialty ingredients business -
News Lonza to expand bioconjugation capabilities at Visp site
CDMO will add two production suites for clinical and commercial supply and extend lab space to double analytical and process development capacity -
News Lonza expands development and manufacturing capabilities at Bend site
The company will invest in dedicated early-phase development and cGMP manufacturing to help customers rapidly advance challenging molecules -
News Altimmune signs up Lonza to manufacture intranasal COVID-19 vaccine
Altimmune adds Lonza as a manufacturing partner for supply of AdCOVID, its single-dose intranasal vaccine candidate for COVID-19. -
News Ingredients and Formulation - evolving opportunities and challenges
Ingredients are the lifeblood of the pharmaceutical and nutraceutical industries. They connect today’s cutting-edge medicine and consumer products makers with their origins in the chemicals sector of the late nineteenth century. While ingredients play a major part in determining whether a pharmaceutical or nutraceutical product is effective, they do not do it alone. How an ingredient is formulated is critical to the success of a prescription medicine or OTC product, both therapeutically and from a commercial standpoint. -
News Ingredients and Formulation - evolving opportunities and challenges
Ingredients are the lifeblood of the pharmaceutical and nutraceutical industries. They connect today’s cutting-edge medicine and consumer products makers with their origins in the chemicals sector of the late nineteenth century. While ingredients play a major part in determining whether a pharmaceutical or nutraceutical product is effective, they do not do it alone. How an ingredient is formulated is critical to the success of a prescription medicine or OTC product, both therapeutically and from a commercial standpoint. -
News Technical article: Particle engineering approaches for dry powder inhalers
As respiratory diseases continue to be a leading cause of death and disabilities across the globe, inhalation drug products play a significant role in treatments. Particle engineering innovations can help keep these medicines affordable and patient-centric.
In this article read how choosing the right technology for particle engineering helps life-saving therapies reach patients faster. Understanding API properties, target drug profiles and potential risks all play a role in choosing the right particle engineering technology, such as spray drying or micronization.
-
News On-demand webinar: Engineering approaches to respiratory drug delivery: Mannitol case study
Both spray drying and jet milling are commercially viable engineering processes for the development of respirable drug products.
This case study explores the material and performance properties of mannitol, spray-dried and jet-milled, for respiratory delivery. The head to head comparison reveals opportunities and risks for designing a product based on each approach. -
News On-demand webinar: Technical considerations for capsule-based inhalation product development
Drug delivery to the lung is an important and growing field of research for both local and systemic treatments. Efficient nasal and pulmonary drug delivery requires precise particle size and density control.
Lonza Pharma & Biotech has premier particle engineering capabilities with which to meet the exacting formulation requirements for dry powder inhaler (DPI) applications, utilizing both spray drying and particle size reduction technologies. These capabilities extend to the ability to control particle properties to interact positively with both capsule and delivery devices, making Lonza a preferred service partner for DPI products. -
News Inhalation formulation services with premier particle engineering platform
Lonza Pharma & Biotech provides integrated DPI (dry powder inhaler) development services to advance molecules, drawing on 25 years of experience in particle engineering, formulation design, and encapsulation techniques.
Our premier particle engineering technologies services, utilizing both jet milling and spray drying, are tailored to your molecule, target product profile and delivery device. Dedicated DPI formulation, analytical and manufacturing teams ensure that development timelines are met while minimizing program risk and complexity. -
News Lonza enhances product line to tackle concerns around bias during blinded clinical trials
The cost-effective capsules incorporate anti-tampering measures to ensure they comply with blinding regulations.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










.jpg)


.jpg)












-file142925.png)

-file142804.png)

-file142748.png)



-file142750.png)

-file142759.png)









.jpg)



%20(3).jpg)