High Potent Manufacturing
Product Description
Bluepharma Industria Farmaceutica S.A.

-
PT
-
2015On CPHI since
-
5Certificates
-
500 - 999Employees
Company types
Primary activities
Categories
Specifications
Bluepharma Industria Farmaceutica S.A.

-
PT
-
2015On CPHI since
-
5Certificates
-
500 - 999Employees
Company types
Primary activities
More Products from Bluepharma Industria Farmaceutica S.A. (4)
-
Product Clinical Manufacturing
.Integrated drug development service (CRO/CDMO) including clinical manufacturing and management.
The range of activities offered by Bluepharma includes:
- Clinical material cGMP manufacturing
- Clinical supply management
- clinical material
- placebo
- ... -
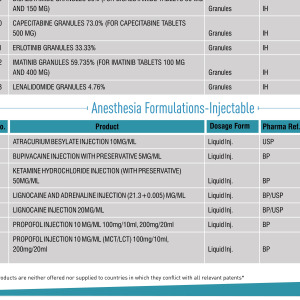
Product Complex Sterile Formulations Contract Development
+25 Years of accumulated experience in Lipid and polymer-based formulations.
Regarding Complex Injectables, Bluepharma's focus is on Lipid-based, polymer-based, and Long-acting injectable (LAI) formulations. Bluepharma allies the scientific expertise with the technical capability to ensure the success... -
Product Drug Delivery Platforms
Modified release, hot-melt extrusion, ODT, oral thin films, oral sprays, and polymer-based solutions,
Our range of technologies covers solid dosage forms (IR and ER), polymer-based solutions, solid dispersions (hot-melt extrusion), oral sprays, oral thin films, and complex injectables ... -
Product Pharmaceutical development (CDMO) & Exclusive portfolio
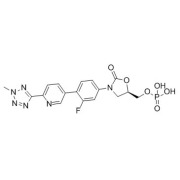
From research, pre-formulation, and formulation development, up to commercial manufacturing (including HPAPI).
Bluepharma offers an integrated drug development service (CDMO) from research, pre-formulation, and formulation development, up to commercial manufacturing ...
Bluepharma Industria Farmaceutica S.A. resources (5)
-
Video Bluepharma´s Presentation
Bluepharma is a Portuguese Group with more 20 years, more than 700 employees and 20 innovative companies that cover all stages of the pharmaceutical industry value chain of the drug, from R&D to the market, imposing itself for the excellence of its production unit, the quality and training of its employees and the experience and dynamism of its management team. -
Brochure Differentiated Portfolio
Unlock a world of complex generics & value-added products in our differentiated portfolio, serving the global market. Explore excellence today! -
Brochure Platforms for Value Added Medicines (VAM)
Discover our innovative portfolio & collaborative approach to bring your ideas to life. Explore value-added solutions with us today! -
Brochure Diversified and exclusive portfolio
Development and Licensing Opportunities.
Due to our extensive international track record (successfully inspected by FDA, EMA, ANVISA, among others), we collaborate globally with major pharmaceutical companies, by licensing out our own technology or distributing our own brand worldwide, namely in the United States, Europe, Middle East, APAC, China and LATAM. -
Video “Reflections in Future”, Bluepharma´s 20 years celebration photographic exhibition.
www.bluepharma20anos.pt
Bluepharma turns 20 years in 2021! 20 years that have passed like a flash.
To honor all employees who have been part of these two decades, Bluepharma has prepared a photographic exhibition entitled “Reflections in Future”, with photos by Pedro Medeiros.
“Reflections in Future” is an exhibition with 20 photographs that celebrates the 20th Anniversary of Bluepharma, the Portuguese pharmaceutical company that was born in Coimbra and that, step by step, went from a unit with 58 workers to a group of 20 companies, with 700 employees, including 260 researchers.
With all the authenticity achieved by the telephoto lens, centered on People, Investment, Research and Quality, we present you the challenge posed in each procedure.
This exhibition can also be seen as a dynamic and didactic display of the complexity of the pharmaceutical industry's value chain.
From Research & Development to kno...
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance














































-comp273426.jpg)