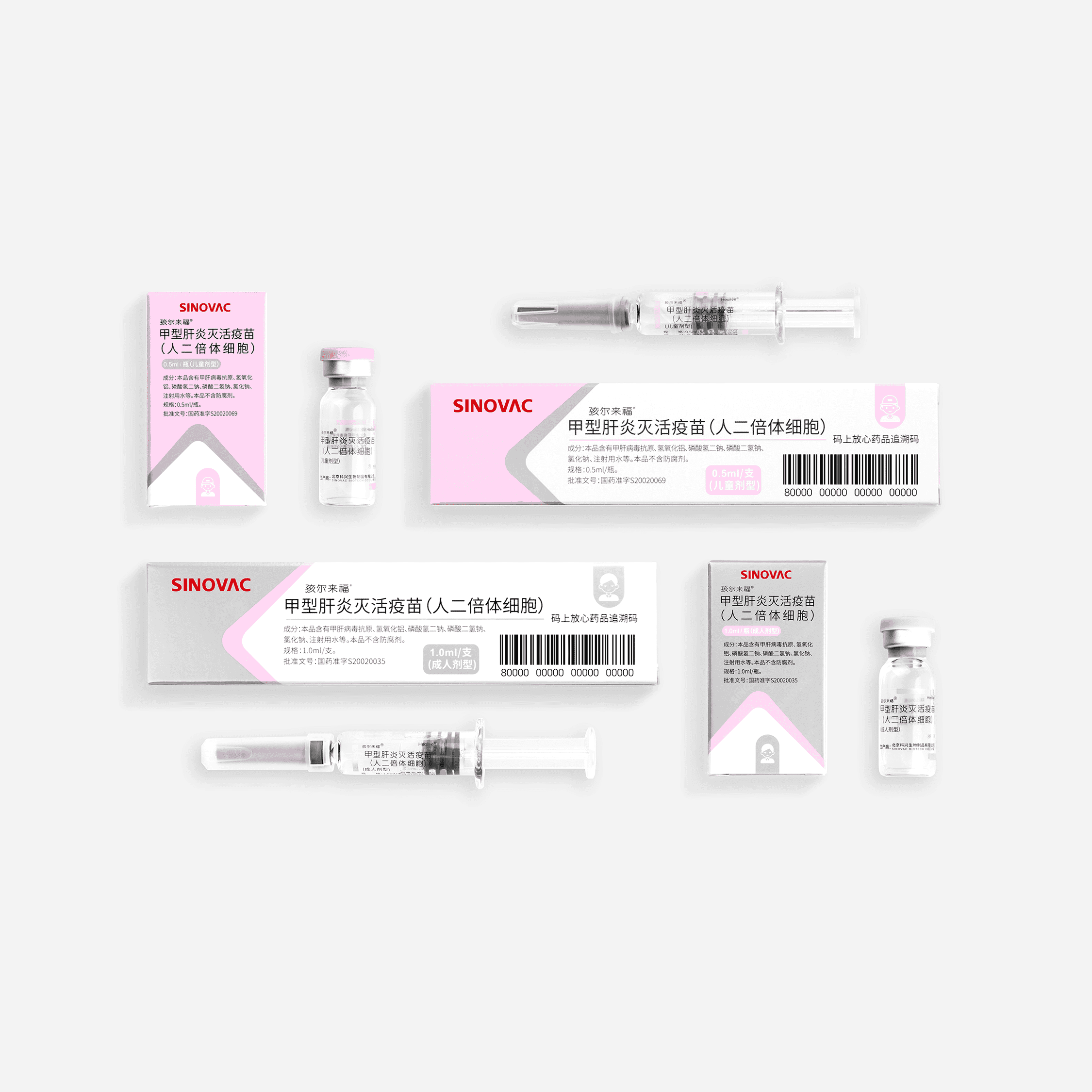
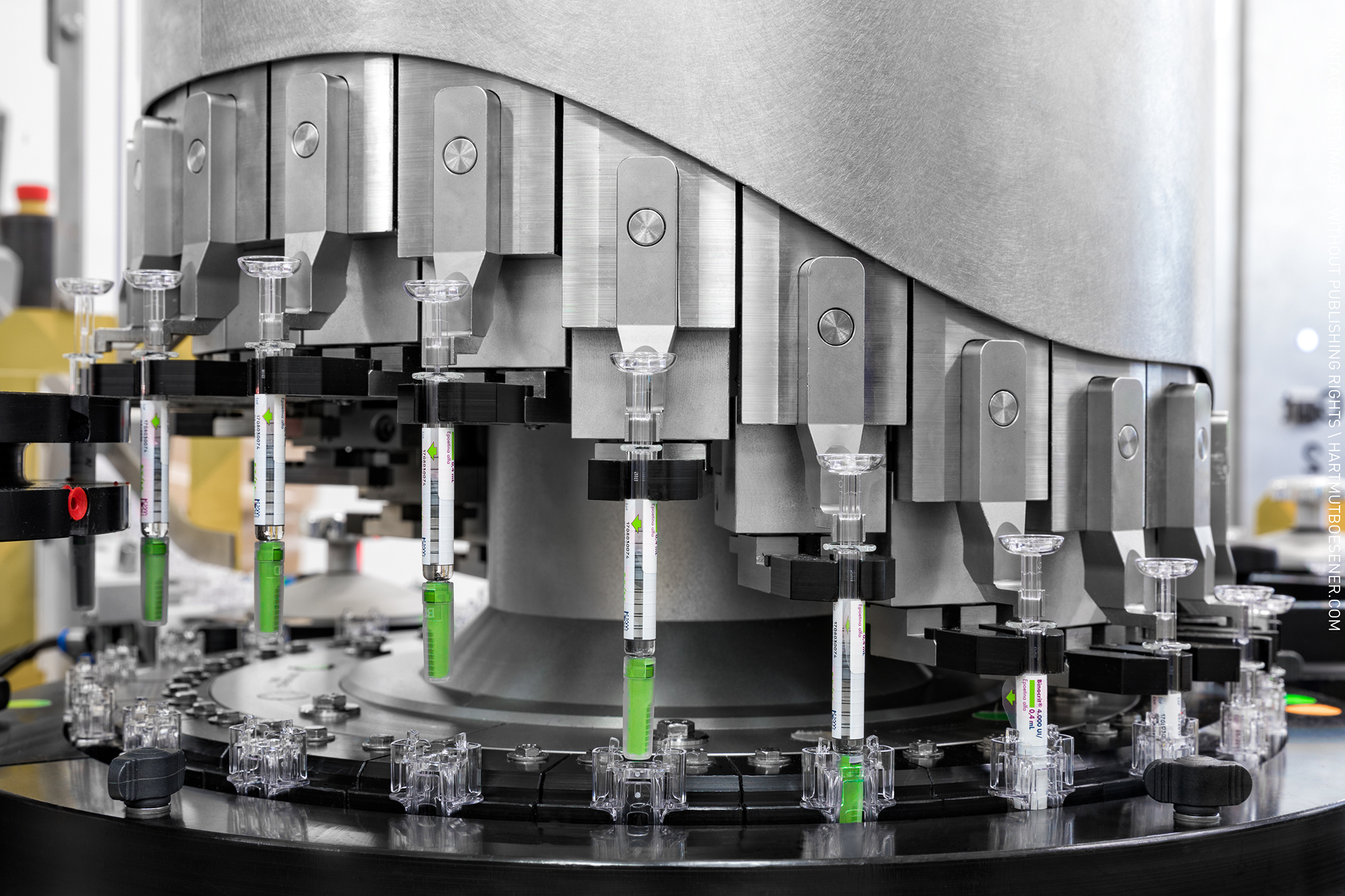
Hepatitis A Vaccine (Human Diploid Cell), Inactivated - Healive®
Product Description
Sinovac Biotech Ltd.

-
CN
-
2018On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
Categories
Specifications
Sinovac Biotech Ltd.

-
CN
-
2018On CPHI since
-
2Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Sinovac Biotech Ltd. (3)
-
Product Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains
Launched in 2021.
Prequalified by the World Health Organization.
Manufacturing process is result of technology transfer from the WHO and Netherlands-based lntravacc.
Good safety with zero preservatives and antibiotics.
Read more on: http://ww... -
Product Influenza Vaccine (Split Virion), lnactivated – Anflu®
Influenza Vaccine (Split Virion), lnactivated – Anflu®
Pandemic Influenza Vaccine, lnactivated, Adjuvanted – Panflu®
H1N1 Influenza A Vaccine (Split Virion), lnactivated – Panflu.1®
Influenza Vaccine (Split Virion), lnactivated, Quadrivalent – TetrAnflu®
Anflu®, preservative-free ... -
Product SYN023 (Zamerovimab and Mazorelvimab Injection)
Zamerovimab and Mazorelvimab Injection (SYN023) is the World’s First Humanized mAb Cocktail against Rabies. It has approved in China since 2024.
Sinovac Biotech Ltd. resources (2)
-
News SINOVAC Named BogotáBio Exclusive Partner, Fueling New Vaccine Advances in Colombia
Sinovac Biotech Ltd. ("SINOVAC" or the "Company") (NASDAQ: SVA), a leading provider of biopharmaceutical products in China, today announced it has been named the exclusive strategic partner of BogotáBio, which will break ground as the first local human vaccine production facility to be established in Colombia’s capital city of Bogota.
-
Brochure SINOVAC Vaccines leaflets
Sinovac Biotech Ltd. (SINOVAC) is a China-based biopharmaceutical company that focuses on the R&D, manufacturing, and commercialization of vaccines that protect against human infectious diseases.
SINOVAC's product portfolio includes vaccines against COVID-19, enterovirus 71 (EV71) infected Hand-Foot-Mouth disease (HFMD), hepatitis A, varicella, influenza, poliomyelitis, pneumococcal disease, and mumps.
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance