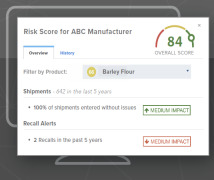
GMP-Support: Qualification / Validation / Consulting

Product Description
Quasaar GmbH

-
DE
-
2017On CPHI since
-
2Certificates
-
25 - 49Employees
Company types
Categories
Quasaar GmbH

-
DE
-
2017On CPHI since
-
2Certificates
-
25 - 49Employees
Company types
More Products from Quasaar GmbH (5)
-
Product Stability and Ageing Studies
The Quasaar Stability Centre offers flexible and large-volume storage facilities, which also allow the storage of voluminous packaging units (pallets) up to bulk goods. In addition to conducting ICH studies, it is also possible to place ageing studies, e.g. of medical devices. In this context, leakage and ... -
Product Analytical services
Analytical services
In addition to the basic product quality control, the following connected services are offered, which are required throughout the product development or before the market launch of the respective products:
GMP for excipients, risk assessment... -
Product Quality Control: Excipients / API / Finished Products
Quality Control: Excipients / API / Finished Products
The service profile of Quasaar GmbH primarily includes the quality control of pharmaceuticals (human and veterinary), active ingredients, raw materials and excipients.
Hereby the effectiveness and safety of the product for the prote... -
Product Stability/Ageing Studies
Stability/Ageing Studies: 500 m3 to 2000 m3 ICH-Climates
The Quasaar Stability Centre offers flexible and large-volume storage facilities, which also allow the storage of voluminous packaging units (pallets) up to bulk goods.
In addition to conducting ICH studies, it is also pos... -
Product Technology Transfer
Technology Transfer
Methods transfer, risk analysis and robustness investigations,
seamless transfer from development stages into the regulated GMP environmentseamless transfer from development stages into the regulated GMP environment
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance








































































-comp296622.jpg)