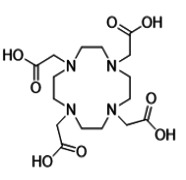
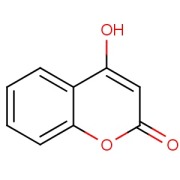
Gadoterate Meglumine

Product Description
Anhui Poly Pharm Co.,Ltd

-
CN
-
2023On CPHI since
-
500 - 999Employees
Company types
Categories
Specifications
Anhui Poly Pharm Co.,Ltd

-
CN
-
2023On CPHI since
-
500 - 999Employees
Company types
More Products from Anhui Poly Pharm Co.,Ltd (4)
-
Product Cyclophosphamide
CDE: A DMF No.: 037154 CEP:under review , Q3 2024ASMF available -
Product Gadobutrol
CDE: I DMF No.: 039149 CEP/ASMF: Q2 2025 -
Product Glycopyrrolate
CDE: A DMF No.: 035446 R0-CEP 2021-154-Rev 00 -
Product Semaglutide
DMF/ASMF: Q3 2024 CEP: Q3 2024
Anhui Poly Pharm Co.,Ltd resources (1)
-
News API production lines respectively for Gadoterate Meglumineand Cyclophosphamide passed the FDA on-site inspection.
In 2023, our API production lines respectively for Gadoterate Meglumineand Cyclophosphamide passed the FDA on-site inspection.
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance















































%20(1)%20(1)-comp246058.png)