Freudenberg expands biopharma product portfolio with Single-use Assemblies
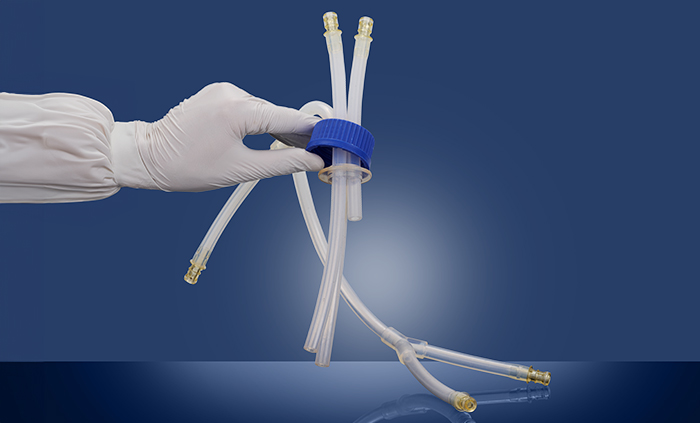
custom single-use assemblies and bottle cap assemblies
Freudenberg's Biopharma division has expanded fluid handling capabilities with the addition of custom single-use assemblies
August 1, 2024 - Freudenberg Medical has expanded its biopharma product portfolio to include custom single-use assemblies which combine Freudenberg’s expertise in silicone extrusion and molding. New offerings include, but are not limited to, single-use y-connector manifolds, tubing assemblies, and bottle cap assemblies with multiple inlets/outlets. Bottlecap assemblies with y-connector tubing are commonly used in lab or cGMP bioprocesses for critical fluid transfer applications.
All assemblies are cleanroom manufactured according to customer specifications. Y-connector overmolds guarantee an unobstructed flow within a critical fluid path and will prevent leaks commonly seen with standard fluid couplings, fittings, and connectors. Validated production processes with proven process capabilities will continuously ensure consistent overmolding of y-manifolds on customer-specified tube ends.
Freudenberg’s expanded biopharma solutions complement the existing PharmaFocus® Premium line of high purity, platinum-cured silicone tubing and sanitary ends for single-use systems. Freudenberg’s tubing, molded components and systems are used in bioprocessing, biotechnology, lab processes, GMP manufacturing, small batch processes, cell & gene therapy, and the production of mRNA, biosimilars and regenerative proteins.

Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance