Formulation of lyophilized products
Product Description
Comser

-
ES
-
2022On CPHI since
-
1Certificates
-
25 - 49Employees
Company types
Primary activities
Categories
Specifications
Comser

-
ES
-
2022On CPHI since
-
1Certificates
-
25 - 49Employees
Company types
Primary activities
More Products from Comser (7)
-
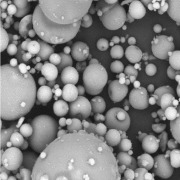
Product Formulation of lyophilized products
A correct formulation means greater stability of the active ingredient and provides protection against the stress suffered during the lyophilization process itself.
The selection of the appropriate excipients is really important in the development of a freeze-dried product. -
Product Thermal characterization of the product
Having information on the thermodynamic behavior our formula is critical to identify the critical product temperatures. At Comser, we have the following technology to characterize the product:
Freeze Drying Microscope (FDM)
Differential Scanning Calorimetry (DSC)
-
Product Lyophilization Process Development (Quality By Design approach)
With the aim of obtaining a product that meets the critical quality attributes, but with the most optimized freeze-drying process.
Our focus is the total control of the process. We develop freeze-drying processes in a previously defined design space: Quality By Design approach. -
Product Scale-Up and Transfer of lyophilization processes
From the laboratory to the industry.
The key to a correct Scale-Up and Transfer of lyophilization processes is the knowledge of the developed product, as well as the control of the process and the capabilities of the final equipment. -
Product Theoretical-practical WORKSHOP on lyophilization (3 full days)
The format of this course is hybrid, you can attend in person or online.
Next edition: November 2022 (in Spanish); May 2023 (in English) -
Product Lyophilization Process Development (Quality By Design approach)
With the aim of obtaining a product that meets the critical quality attributes, but with the most optimized freeze-drying process.
Our focus is the total control of the process. We develop freeze-drying processes in a previously defined design space: Quality By Design approach. -
Product Thermal characterization of the product
Having information on the thermodynamic behaviour of our formula is critical to identify the critical product temperatures. At Comser, we have the following technology to characterize the product:
- Freeze Drying Microscope (FDM)
- Differential Scanning Calorimetry (DSC)
And with such,...
Comser resources (2)
-
Brochure Comser High Value Services
Comser High Value Services Catalogues, broken down into three categories:
- Overview of the whole Comser's value added services
- Formulation & Lyophilization contract services
- GMP Qualification & Validation of critical machinery and processes in aseptic environment -
Brochure Comser High Value Services
Comser High Value Services Catalogues, broken down into three categories:
- Overview of the whole Comser's value added services
- Formulation & Lyophilization contract services
- GMP Qualification & Validation of critical machinery and processes in aseptic environment
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance



















.jpg)

































-comp246544.jpg)