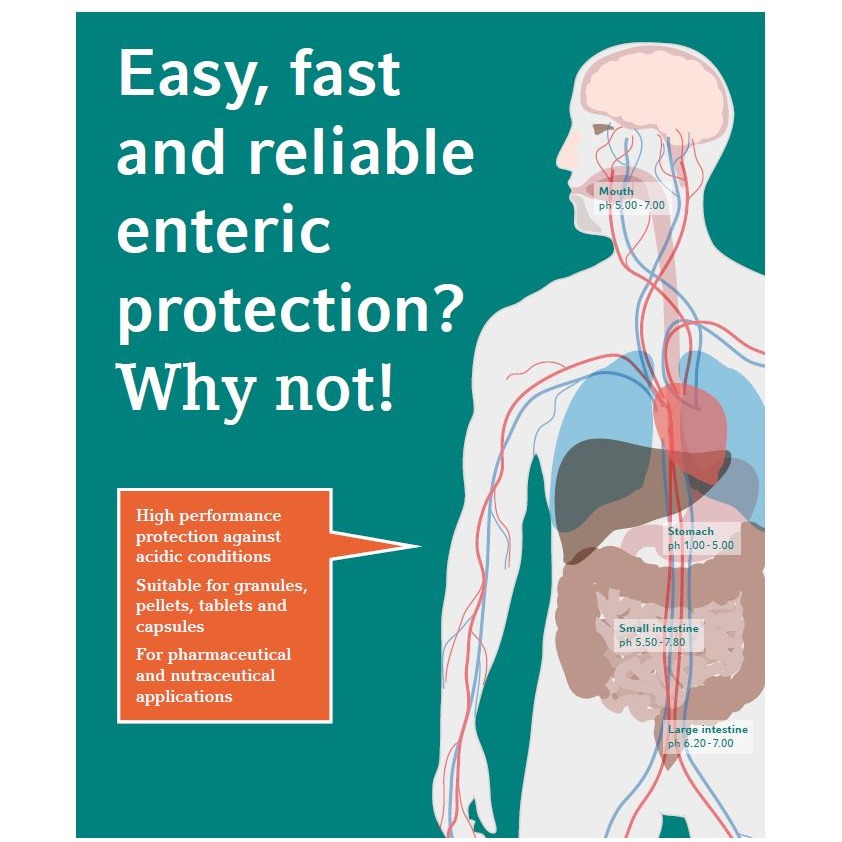
Formulation Development service

Product Description
UPM Pharmaceuticals, Inc.

-
US
-
2017On CPHI since
Company types
Categories
Specifications
UPM Pharmaceuticals, Inc.

-
US
-
2017On CPHI since
Company types
More Products from UPM Pharmaceuticals, Inc. (5)
-
Product Analytical Services
Upm pharmaceuticals offers analytical services. It includes uplc / hplc capability, dissolution testing, full ich stability conditions, microbial testing, purity testing, impurity identification, particle size determination by laser, excipient, compatibility testing, solubility screening, hygroscopicity st... -
Product Clinical Manufacturing Services
Upm pharmaceuticals offers clinical manufacturing services. It processes shorten drug development time and speeds time to the clinic with: direct api fill into capsules, single or bi-layered tableting, encapsulation and over-encapsulation, encapsulation of powders, granules, tablets, and combinations, coat... -
Product Commercial Manufacturing
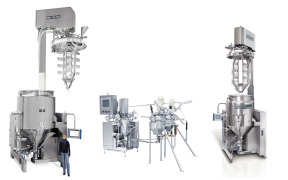
Upm pharmaceuticals offers commercial manufacturing. It encompasses 475,000 square feet on almost seven acres. This facility provides upm with large scale commercial capabilities for manufacturing and packaging of solid oral dosage tablets and capsules, as well as semi-solid manufacturing of creams and oin... -
Product Packaging Services
Upm pharmaceuticals offers packaging services. It has packaging capabilities for filling of tablets and capsules into bottles; and filling of semi-solids into jars and tubes. It has an additional site with 300,000 square feet of warehousing space. With our highly-trained staff, detailed scheduling process,... -
Product Proof of concept services
Upm pharmaceuticals offers proof of concept services. It is widely recognized for speed, precision and innovative thought across a full spectrum of studies. it evaluate a number of parameters for development and manufacture of specific api (active pharmaceutical ingredient) dosage forms based. Brevi-batch ...
UPM Pharmaceuticals, Inc. resources (2)
-
Brochure UPM Corporate Brochure
UPM is a full service CDMO.From Concept to Commercialization for solid oral and semi-solid dosage forms
Frequently Viewed Together
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance






















































































![NexArni 50 [Valsartan with Sacubitril Tablets]](/46/product/129/06/57/picture.jpeg)



