- Home
- Laurus Labs Limited
- Finished Dosage Form
CPHI Online is the largest global marketplace in the pharma ingredients industry
-
Products0
-
Companies0
-
Articles0
-
Events0
-
Webinars0
Finished Dosage Form

Product Description
Laurus is forward integrating into finished dosage forms (FDF) to create more value, generated through its cost effective processes and large capacities in the API business, for its customers.
The Finished Dosage Form Development center is an integral part of Laurus R&D, comprising of Formulation and Analytical Research, Clinical Pharmacodynamics and Pharmacokinetics, Regulatory Affairs, Packaging Development, Intellectual Property Management and Developmental QA departments. Laurus has dedicated formulation research labs, laboratory scale clinical supply facility & analytical research labs capable of developing different types of dosage form.
In parallel, Laurus is establishing state of art oral finished dosage facilities conforming to international regulatory standards. The facility will have a pilot plant and initial commercial capacity of 1 billion units, expanding to 5 billion units over time, with investment of US$50million.
We are building on Laurus R&D’s strengths in chemistry research, process chemistry, active pharmaceutical ingredient production and regulatory filings to forward integrate to FDF Business. FDF business is committed to strengthen and further enhance Laurus’ vision of becoming a leading player in offering integrated solutions to global pharmaceutical needs.
Laurus Labs Limited

-
GB
-
2017On CPHI since
-
-
-
37/39 Southgate Street, Winchester, SO23 9EH, Hants, United Kingdom
-

-
Dhruv AroraHyderabad, India
-
-
-
-
-
-
-
-
-
Ian JacksonHyderabad, India
-
-
Eridona MehHyderabad, India
-
MARTYN OLIVER JAMES PECKWinchester, United Kingdom
-
Laurus Labs Limited

-
GB
-
2017On CPHI since
-
-
-
37/39 Southgate Street, Winchester, SO23 9EH, Hants, United Kingdom
-

-
Dhruv AroraHyderabad, India
-
-
-
-
-
-
-
-
-
Ian JacksonHyderabad, India
-
-
Eridona MehHyderabad, India
-
MARTYN OLIVER JAMES PECKWinchester, United Kingdom
-
More Products from Laurus Labs Limited (2)
-
Product Active Pharmaceutical Ingredients
Building on our strong capabilities in chemical development and manufacturing, Laurus Labs has developed an in-house range of APIs and related intermediates. Focusing on chemistries where we can make a difference, Laurus combined innovation with efficiency by developing cost effective processes w... -
Product Laurus Synthesis
The core-strength of Laurus is its innovative, robust & scalable chemistry - making use of which, multiple developmental candidates of client organizations have been handled at all stages of drug lifecycle right from route identification through tocustom synthesis and from early clinical...
Frequently Viewed Together
-
Product Tramadol HCl
Grünenthal GmbH offers a selected range of products which includes Tramadol HCl, as API, bulk and/ or finished dosage form. Please contact us for more information.
-
Product Polyglykol
Effective and high-purity ingredients including a complete range of Polyglykols (Macrogols) our universally applicable pharmaceutical polymers, with various molecular weights, supported by CEP certification and DMF type II documentation.
-
Product Oral Dosage Forms
We have extensive experience and know-how in the development of oral dosage forms containing spray dried powders. Tablets and capsules are the most common dry powder dosage form. However, other dosage forms such as sachets (bulk powders), vials (for reconstitution) have also be developed by the Upperton team.
-
Product BLISTER MACHINES SERIES "TF"
Automatic blister machines for different production needs (from 100 up to 600 blisters/minute).Main stations driven by brushless motors, forming system made by plates (hot or cold), sealing performed by roll.Size change over very quick and simple, reduced overall dimensions.
-
Product Oral solids
Our experienced formulation development teams support commercial development and manufacturing strategies across a wide range of drug programs.
Your most trusted partner for oral solids:
- Extensive knowledge in highly potent APIs
- Resilient and reliable supply chains supported by our global ne...
-
Product Drum Laser - Tablet & Capsule Marking & Drilling Machine
Powerful multi-lane Class 1 Laser drilling and marking / writing / etching machine that provides single or double-sided laser drilling or laser writing for mid-sized pharmaceutical, nutraceutical or confectionery productions. With output rates of up to 180,000/hour, the Drum Laser works with tablets, capsu...
-
Product Cephalosporins Finished Products
<Injection> Cefazolin Sodium 1g Cefmetazole Sodium 1g, 2g Cefotaxime Sodium 1g Ceftazidime Hydrate 0.5g, 1g, 2g Ceftizoxime Sodium 1g, 2g Cefoperazone Sodium 1g, 2g Cefotiam HCl 1g Ceftriaxone Sodium 1g Cefepime HCl 1g *Flomoxef Sodium 0.5g, 1g *Cefamandole Nafate 1g, 2g *Ceftaroline Fosamil 400mg...
-
Product CordenPharma Highly Potent & Oncology Platform
One Source for Highly Potent & Oncological Products
• Integrated Supply: APIs & Oral / Sterile Drug Products • State-of-the-art facilities able to handle API and Drug Product of the highest potency • Development, clinical trial and commercial manufacturing • Full-service offering includi...
-
Product Walk in Cooling Cabinet.
The Walk-In Cooling Cabinets / cold rooms are designed according to ICH guidelines, WHO, MCA and USFDA requirements to maintain uniform conditions. They are superior in airflow distribution, temperature control technology, cabinet construction and are manufactured as per cGMP regulations.
Biggest Walk...
-
Product Pharmaceutical and Biological Products Contract Development and Manufact...
Bora Pharmaceuticals is a premier international contract development and manufacturing organization (CDMO). Our six state-of-the-art cGMP manufacturing facilities across Asia and North America deliver to more than 100 markets around the world. Our sites have the highest industry standards for quality, dev...
-
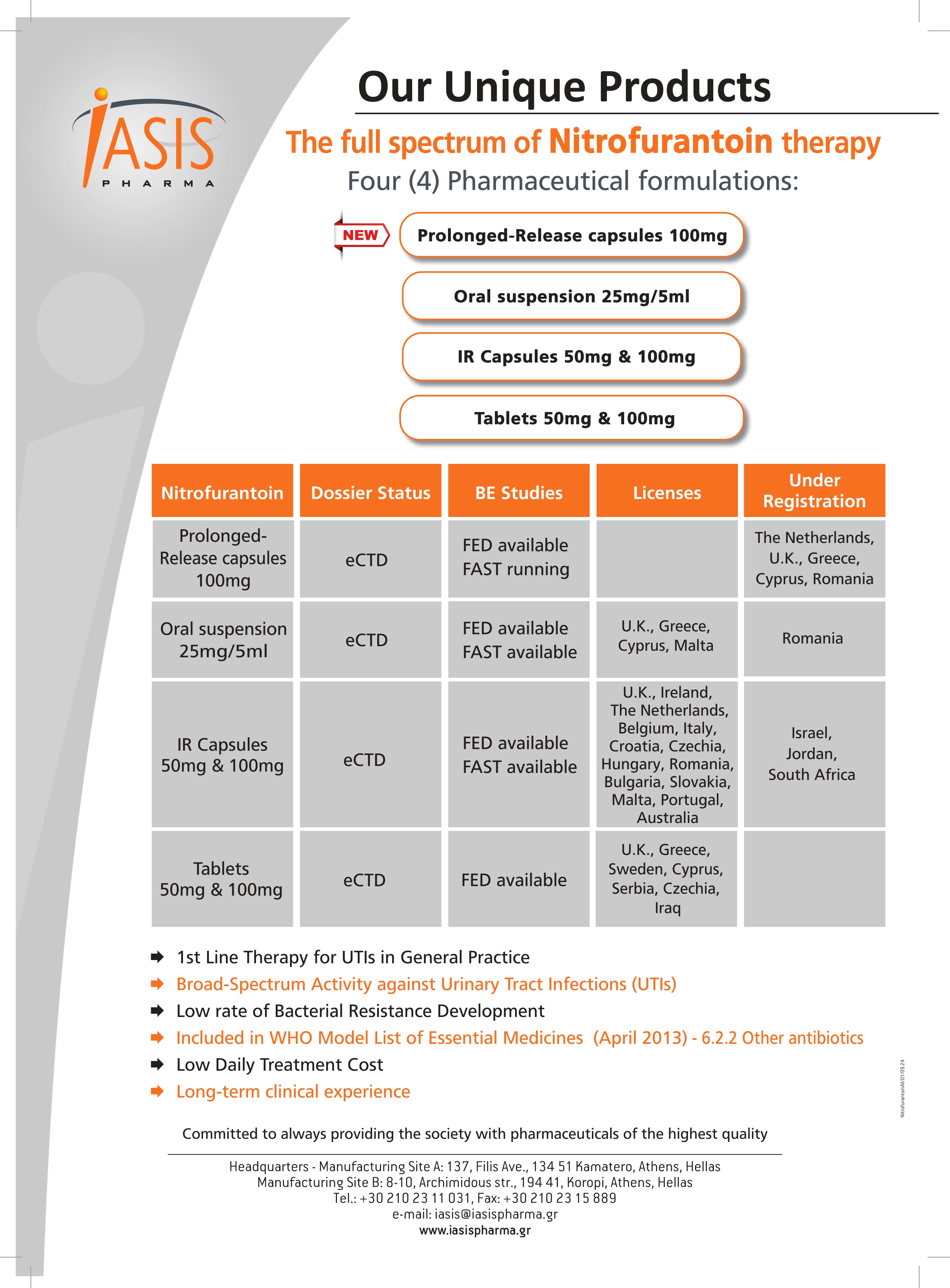
Product FUROLIN® Portfolio
Iasis Pharma S.A. provides a wide range of products which includes Furolin® (Active ingredient; nitrofurantoin). It belongs to antimicrobial agents category. Active ingredient: nitrofurantoin. Contact us for more information.

-
Product APETAMIN
Til healthcare pvt ltd provides wide range of appetite stimulant which includes apetamin. Feature: cyproheptadine + multivitamin + lysine. Dose: 2 mg. Dosage form: syrup. Shelf life: 18. Packaging: 120 & 200 ml. Contact us for more informations.
-
Product Solid
Through three manufacturing sites, Famar offers a wide variety of oral solid product forms including specialty types such as multi-layer tablets.
Famar: • has an expertise with hard gelatine capsule and the technology to cover all capsule sizes based on its customer needs.
• offers manu...
-
Product Shedir Products
With more than 150 products, Shedir is fully capable of satisfying the needs of every potential customer.
Our products range from Food Supplements to Medical Devices Class I, Cosmeceuticals and Pharmaceutical Drugs, all of them produced in plants complying to the highest standards of the...
-
Product Oral Drug Delivery - May/Jun 2023 - Issue 148
Available now to read online at: https://www.ondrugdelivery.com Contents: Strategies for Streamlining the Development of Complex Oral Drug Products Aruna Railkar, Senior Drug Development Consultant. Quotient Sciences Modified-Release Microspheres: Maximising Coating Integrity and Optimising Release Jona...
-
Product CDMO- Generics/OTCs in Tablets, Capsules, Granules...
FDA approved facility in China providing Contract Development and Manufacturing Services in Various Dosage Forms: • Tablets • Capsules • Granules/Powder for OS • Oral Solutions • Drops • Ointment • Cream • Gel
Applicable for most generics/OTCs and innovative drugs.
-
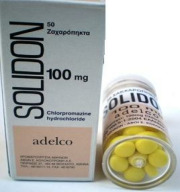
Product Solidon Chlorpromazine 100mg tab
Chlorpromazine is a medication used to manage and treat schizophrenia, bipolar disorder, and acute psychosis. It is a member of the typical antipsychotics or neuroleptic medication category, also known as first-generation antipsychotics.
-
Product products
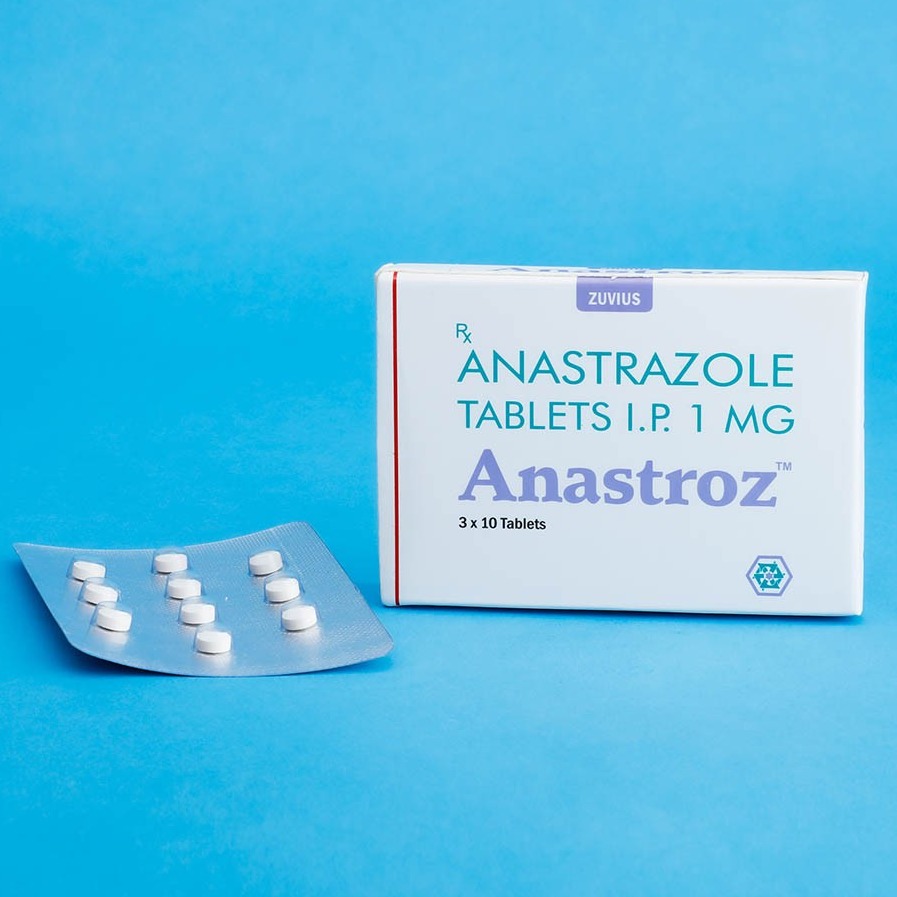
Tablets 1ZefinibGefitinib250mg1 x 10 2CapetazCapecetabine500mg1 x 10 3AnastrozAnastrozole1mg1 x 10 4ZutamTamoxifen20mg1 x 10 5ZuvitrexMethotrexate 2.5mg1 x 10 6ZuvitrexMethotrexate7.5mg1 x 10 7ErlononErlotinib100mg1 x 10 8ErlononErlotinib150mg1 x 10 9ZuvitrozLetrozole...
-
Product Guanfacine Hydrochloride
Guanfacine is indicated for the treatment of Attention Deficit and Hyperactivity Disorder (ADHD) in children and adolescents (6-17 years old) for whom stimulants are not suitable, not tolerated or have been shown to be ineffective.Guanfacine must be used as a part of a comprehensive ADHD treatment programm... -
Product Opadry® Complete Film Coating Systems
Colorcon offers a wide range of film coating products, all of which can be formulated specifically for your application and regulatory requirements. Whether the desired function for your tablet or multiparticulate dosage is immediate release, enteric release and/or sustained release, we have the film coati...
-
Product Finish Dosage Dossiers for Out-Licensing
For our product portfolio please contact: businessdevelopment@pharosgr.gr
-
Product Doliprane
API: Paracetamol
Dosage form: 1g Box of 10 Scored Tablets - 500mg Box of 20 Scored Tablets - 500mg Capsules Box of 16 - Oral solution, Sachets and Suppository.
Therapeutic class: central nervous system.
Indication: treatment symptomatic pain of mild to moderate and / or febrile states.... -
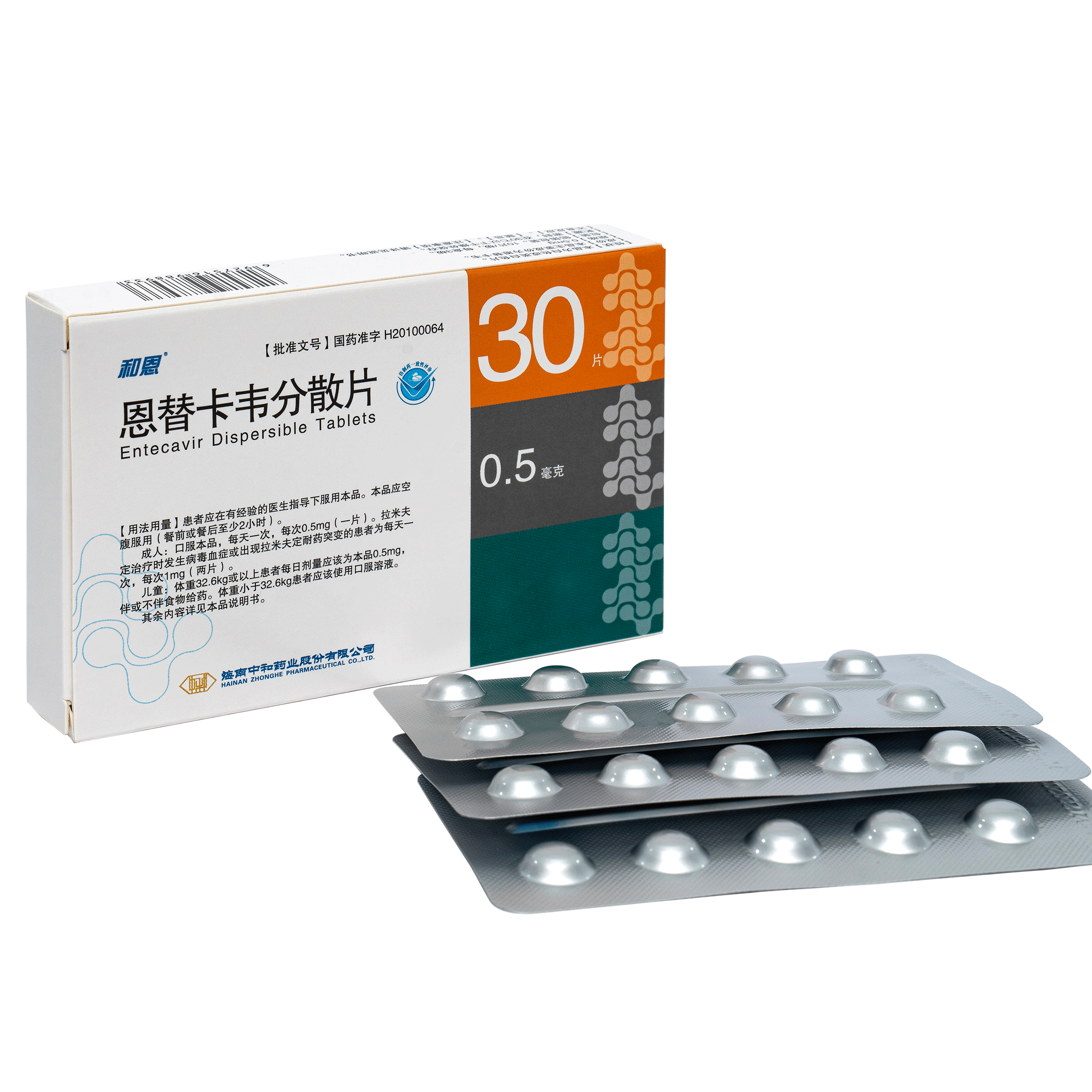
Product Entecavir Dispersible Tablets and Capsules
Strength: 0.5 mg Entecavir is used to treat chronic (long-term) hepatitis B infection (swelling of the liver caused by a virus) in adults and children 2 years of age and older who have liver damage.
-
Product Technologies
Hydroxyapatite Microcapsules • Nutraceuticals and Pharmaceuticals • Circumvention of first pass mechanism with rapid onset of substances that have poor bioavailability when given orally • Bitter substances that need taste-masking (in particular forlow soluble substances) • Targeted release (delayed or s...
-
Product ROTO CUBE
ROTO CUBE – Single-pot processor
Developed in 1984 as a contained solution for the processing of highly pharmaceutical products, ROTO CUBE technology became the benchmark for single-pot process.
ROTO CUBE allows the entire process of any type of product, from the loading of raw...
-
Product Azithromycin Range
Azithromycin Tablets 250mg / 500mg Azithromycin Capsules 500mg Azithromycin Suspension 200mg Per 5ml API is CEP Grade and from our own group company which is USFDA accredited
-
Product Food Supplements
We develop and produce food supplements in these pharmaceutical forms:
Tablets
Syrups
Drops
Oils
Capsules
Sachets
Vials
Soft Capsules
Stick pack
-
Product Via Lattea Tablets
Via Lattea is a food supplement based on dry extracts of Galega, Fenugreek and Fennel. Galega is useful for its galactogoga function (a stimulating action on the production of breast milk).
DOSAGE
Take 1 tablet of Via Lattea per day before main meals with plenty of water during the entire period ...
-
Product Pregabalin Capsules - Gabafine
Gabafine Capsule contains Pregabalin, works by reducing abnormal electrical brain activity, effectively preventing seizures in epilepsy treatment.
Furthermore, it is utilized to address nerve and muscle pain by obstructing pain signals.
Available Strength & Dosage Forms - Preg...
-
Product Acid Protectant Capsule
EMBO CAPS® AP capsules are perfect to encapsulate probiotics, enzymes and other acid sensitive fills. AP Capsule is stronger in acidic environment than regular HPMC capsules.
-
Product VERBAXIB
FOOD SUPPLEMENT Helpful for the treatment of inflammatory state that can lead to joints pain. It contains a patented plant extract titrated in verbascoside, acting as COX-2 selective inhibitor to reduce joint inflammation and reduce the pain in people who suffer from osteoarthritis.
-
Product Functional film coating
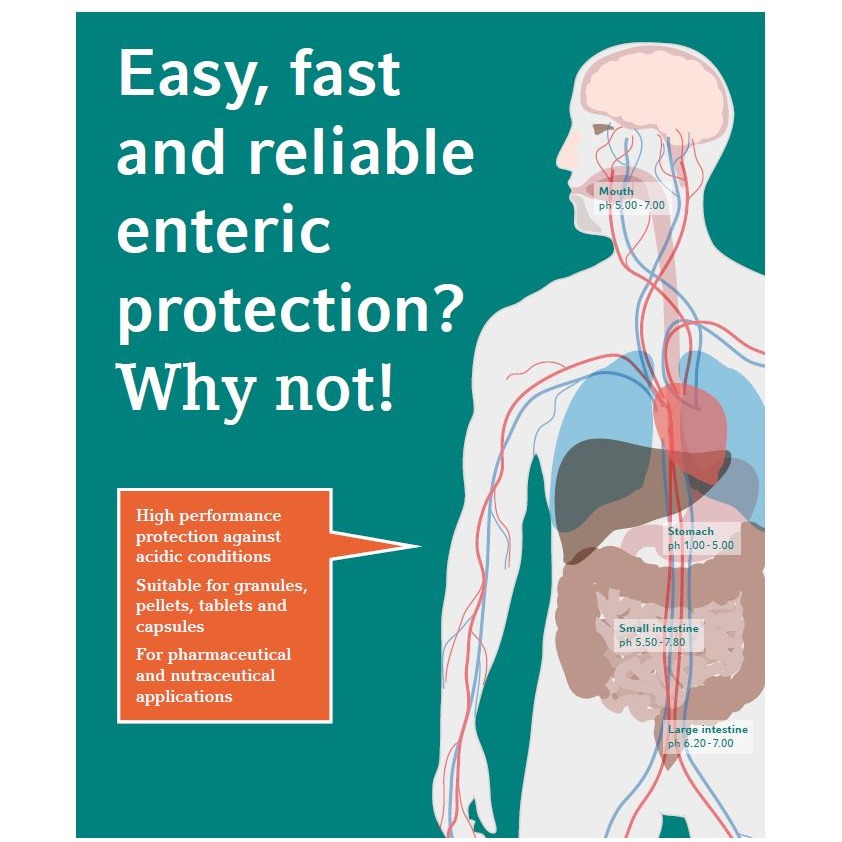
Easy, fast and reliable enteric protection? Why not! - High performance protection against acidic conditions. - Suitable for granules, pellets, tablets and capsules. - For pharmaceutical and nutraceutical applications.
-
Product Nutraceutical Range
1. Vitamin D Supplement 2. Calcium Supplement 3. Iron Supplement 4. Brain Supplement 5. Liver Supplement 6. Immunity Booster Supplement 7. Prenatal Supplement 8. Pre & Pro Biotics 9. Joint Supplement 10. Diabetic Supplement
-
Product Champs Multivitamin Plus Omega-3 (Fruity)
Pyridoxine HCI/Vitamin B6Vitamin E Natural fish oil Vitamin C Vitamin B2 Riboflavin Nicotinamide Vitamin A Vitamin B12 Vitamin D Vitamin B1-comp282059.jpg)
-
Product SPINE HYPO
Automatic Visual Inspection and Sorting of Highly Potent Tablets & Capsules. Sensum SPINE HYPO machine is designed for contained automatic visual inspection and sorting of highly potent tablets and capsules. The system keeps the same functionality, versatility, quality and ease of use of SPINE, but i...
-
Product Immune Support Range
Propolis is known from ancient times for its beneficial properties. Its ingredients help strengthen the body’s natural defense system in order to cope with the symptoms of the common cold. Powerful Manuka Honey provides exceptional nutritional benefits to the organism. Additionally, Echinacea is traditiona...
-
Product Contract Manufacturing Services
As a Swiss pharmaceutical manufacturer, Acino can look back on to a long history in producing oral solid dosage forms for major generic companies at the highest quality possible. Having developed profound know-how within this field, Acino has a well-known track record in manufacturing complex modified...
-
Product Your Pregnancy Softgels
The leading pregnancy food supplement in several very different markets.
Best quality sources of DHA, folate, iron, vitamins and minerals, all developed in a single daily dose. Proven stability at the end of shelf-life - unique in the pregnancy supplement market. With Qfolate – the most bi...
-
Product B-VITAL® totale
B-VITAL totale is constituted by a multivitamin formulation that includes the entire span of B vitamins in doses balanced between them and, in particular, contains 100% of the recommended daily intake levels of folic acid (400 mcg) for pregnant women (Assumption Reference Levels of Nutrients 1996...
-
Product Activated carbon tablets and capsules
We are dedicated to providing high-quality medicinal activated charcoal products and services to our customers around the world. The active pharmaceutical ingredients (APIs) used in our pharmaceutical processing plant meet or exceed the purity profiles in the United States Pharmacopeia and the European Pha...
-
Product Celecoxib
Anwita Drugs & Chemicals Pvt Ltd offers a wide range of capsules which includes celecoxib. Contact us for more information.
-
Product Oral capabilities
With the highest level of technology and the most innovative solutions applied to all manufacturing areas, BSP can fulfill
the most stringent requirements for handling conventional oral dosage forms as well the next generation of anticancer
and cytotoxic products characterized by complex, non con...
-
Product Finished Formulations
We are a leading third-party manufacturer of health supplements and nutraceuticals in private labeling with more than 800 product portfolios, you can make any type of tailor-made products in capsules, or tablets. effervescent, sprays, syrups, drops, sachets, powder, and candies. We have our in-house label...-comp306407.jpg)
-
Product Hovid Ethical & OTC products
Hovid manufacturers & distributes more than 400 products, comprising generic pharmaceuticals, OTC products, health supplements and traditional/consumer products. The therapeutical ranges are below: a.) Cardiovasular management b.) Diabetes management c.)Dermatologicals range d.) Anti-infectives range ...
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance