ERYSAA®

Product Description
Duopharma Biotech Berhad
-comp282059.jpg)
-
MY
-
2019On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
Duopharma Biotech Berhad
-comp282059.jpg)
-
MY
-
2019On CPHI since
-
3Certificates
-
1000 - 4999Employees
Company types
Primary activities
More Products from Duopharma Biotech Berhad (59)
-
Product Mucolex Injection 4mg/2ml
API: Bromhexine HCI -
Product Champs DHA 100mg Softgel
API: Omega Fish Oil -
Product Champs Multivitamin Plus Folic Acid & Taurine (Lychee)
API:ASCORBIC ACID (VITAMIN C) CHOLECALCIFEROL CYANOCOBALAMIN FOLIC ACID NICOTINAMIDE PYRIDOXINE HYDROCHLORIDE RETINYL ACETATE RIBOFLAVIN TAURINE TOCOPHEROL ACETATE, DL-ALPHA THIAMINE MONONITRATE -
Product Champs Multivitamin Plus Iron & Zinc (Pineapple)
API: NICOTINAMIDE RETINYL ACETATE CHOLECALCIFEROL RIBOFLAVIN CYANOCOBALAMIN ASCORBIC ACID (VITAMIN C) TOCOPHERYL ACETATE, DL-ALPHA CARBONYL IRON THIAMINE MONONITRATE ZINC SULFATE MONOHYDRATE PYRIDOXINE HYDROCHLORIDE -
Product Champs Multivitamin Plus Omega-3 (Fruity)
Pyridoxine HCI/Vitamin B6Vitamin E Natural fish oil Vitamin C Vitamin B2 Riboflavin Nicotinamide Vitamin A Vitamin B12 Vitamin D Vitamin B1 -
Product Champs Vitamin C 100mg (Blackcurrant)
API: Ascorbic Acid -
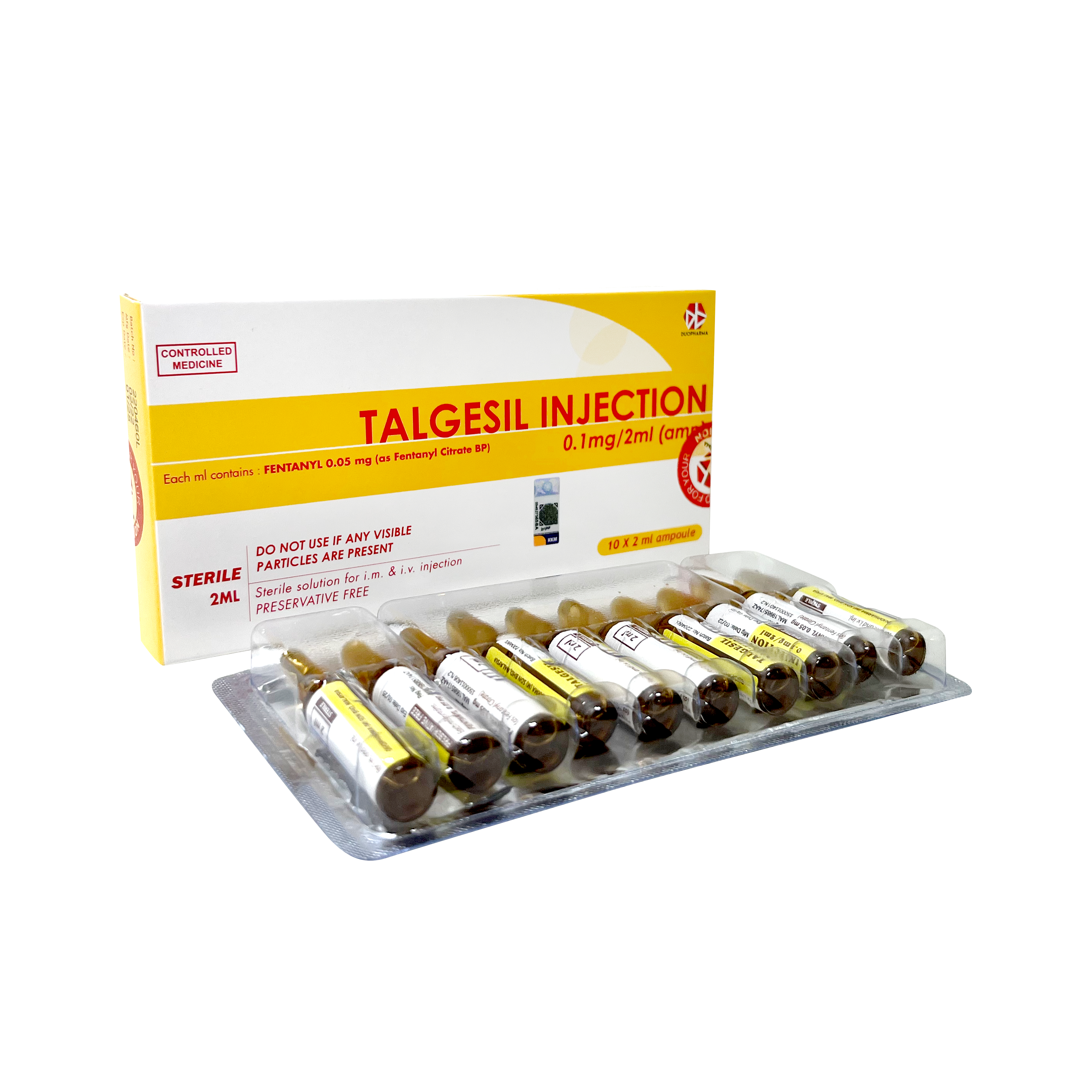
Product Talgesil Inj (Fentanyl Citrade BP)0.1mg/2ml
Fentanyl Citrade BP -
Product Kisan Inj (Phytomenadione) 1mg/ml
API: Phytomenadione 1mg/ml -
Product Zoraxin Suspension 200mg/5ml
API: Acyclovir -
Product Zolterol SR Tablet 100mg
API: Diclofenac Sodium -
Product Zolterol SR Tablet 75mg
API: Diclofenac Sodium -
Product Uphamol Plus Codeine Tablet
API: Paracetamol
Codeine Phosphate
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance