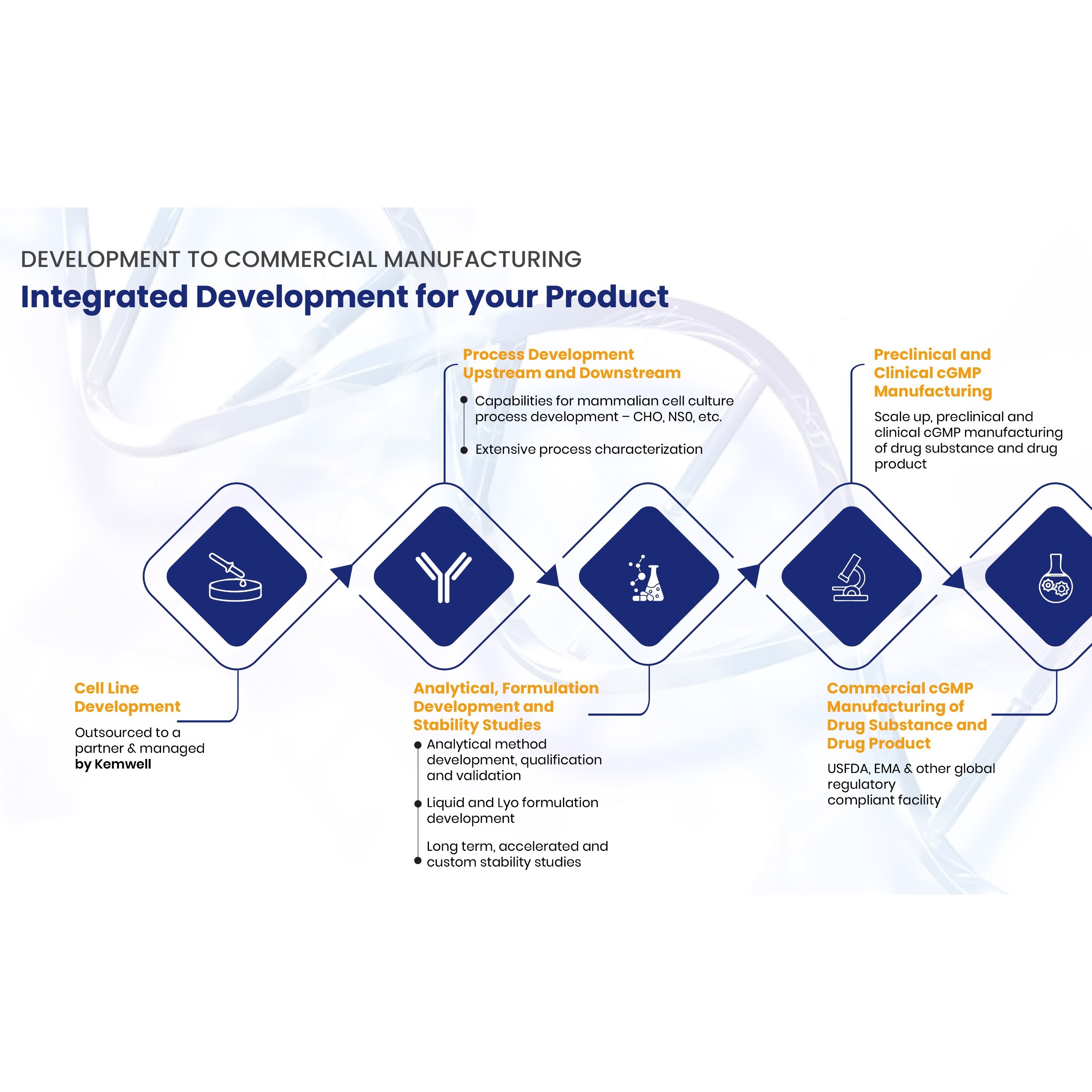
Enerceptant / Etanercept 25mg /50mg PFS

Product Description
AMEGA Biotech

-
AR
-
2019On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
Categories
Specifications
AMEGA Biotech

-
AR
-
2019On CPHI since
-
2Certificates
-
250 - 499Employees
Company types
Primary activities
More Products from AMEGA Biotech (2)
-
Product Somactive LF / Somatropine
Somatropin 5 mg/1,5 ml // 10mg/1,5 ml 15 mg/1,5 ml // 30 mg/3ml For subcutaneous administration Injectable Solution -
Product Follitropin Alpha
Follitropin Alpha
AMEGA Biotech resources (1)
Recommended Products
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance













-comp304625.png)

























-comp316899.png)