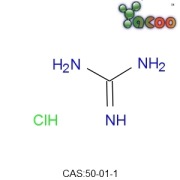
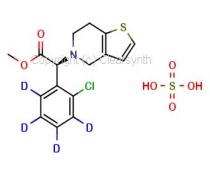
Elemental Impurities

Product Description
Brightlabs

-
NL
-
2021On CPHI since
-
2Certificates
-
25 - 49Employees
Company types
Categories
Specifications
Brightlabs

-
NL
-
2021On CPHI since
-
2Certificates
-
25 - 49Employees
Company types
More Products from Brightlabs (3)
-
Product Herbal Drugs Analysis
Since 2018 Brightlabs possesses an opium exemption from the BMC (Bureau for Medicinal Cannabis), for the analysis of cannabis and related products. The combination of this opium exemption, combined with our GMP accreditation, makes Brightlabs an important partner for the analysis of cannabis and related ma... -
Product Method Development
We are specialist in the method development of analytical methods for analysis of API, Final Drug Products or low level impurities. Usually followed by full validation in accordance to ICH guidelines. -
Product Nitrosamine Analysis
In response to nitrosamines present in pharmaceutical products, the European Medical Agency (EMA) and the US Food and Drug Administration (US FDA) have published requirements and limits related to nitrosamine contaminants. Therefore Brightlabs has developed and validated multiple methods to determine low l...
Brightlabs resources (4)
-
Brochure NDSRI analysis
Brightlabs has developed and validated multiple methods (GC-MS/MS & LC-MS/MS) to determine low level concentrations of Nitrosamines and NDSRIs in Drug Products
Frequently Viewed Together
Recently Visited
Position your company at the heart of the global Pharma industry with a CPHI Online membership
-
Your products and solutions visible to thousands of visitors within the largest Pharma marketplace
-
Generate high-quality, engaged leads for your business, all year round
-
Promote your business as the industry’s thought-leader by hosting your reports, brochures and videos within your profile
-
Your company’s profile boosted at all participating CPHI events
-
An easy-to-use platform with a detailed dashboard showing your leads and performance










.jpg)